Glaucoma
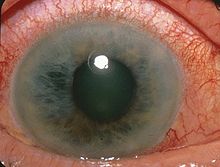
[21] Acute angle-closure glaucoma may further present with corneal edema, engorged conjunctival vessels, and a fixed and dilated pupil on examination.
A study with 1636 persons aged 40-80 who had an intraocular pressure above 24 mmHg in at least one eye, but no indications of eye damages, showed that after five years, 9.5% of the untreated participants and 4.4% of the treated participants had developed glaucomatous symptoms, meaning that only about one in 10 untreated people with elevated intraocular pressure will develop glaucomatous symptoms over that period of time.
[30] Many of these genes are involved in critical cellular processes that are implicated in the development and progression of glaucoma, including regulation of intraocular pressure, retinal ganglion cell health, and optic nerve function.
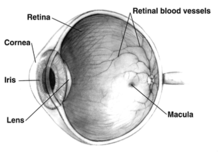
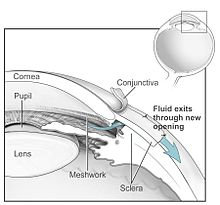
Intraocular pressure is a function of production of liquid aqueous humor by the ciliary processes of the eye, and its drainage through the trabecular meshwork.
[37] In primary angle-closure glaucoma, the iridocorneal angle is narrowed or completely closed, obstructing the flow of aqueous humor to the trabecular meshwork for drainage.
[38][39] Both experimental and clinical studies implicate that oxidative stress plays a role in the pathogenesis of open-angle glaucoma[40] as well as in Alzheimer's disease.
The baseline glaucoma evaluation tests include intraocular pressure measurement by using tonometry, anterior chamber angle assessment by optical coherence tomography, inspecting the drainage angle (gonioscopy), and retinal nerve fiber layer assessment with a fundus examination, measuring corneal thickness (pachymetry), and visual field testing.
Diagnosis is made from physical signs and symptoms - pupils mid-dilated and unresponsive to light, cornea edematous (cloudy), reduced vision, redness, and pain.
This condition is differentiated from malignant glaucoma by the presence of a deep and clear anterior chamber and a lack of aqueous misdirection.
Vascular flow and neurodegenerative theories of glaucomatous optic neuropathy have prompted studies on various neuroprotective therapeutic strategies, including nutritional compounds, some of which may be regarded by clinicians as safe for use now, while others are on trial.
[63][69] Prostaglandin analogues, such as latanoprost, bimatoprost and travoprost, reduce the IOP by increasing the aqueous fluid outflow through the draining angle.
[73][74][75][76] Poor compliance with medications and follow-up visits is a major reason for treatment failure and disease progression in glaucoma patients.
Patient education and communication must be ongoing to sustain successful treatment plans for this lifelong disease with no early symptoms.
Nd:YAG laser peripheral iridotomy (LPI) may be used in patients susceptible to or affected by angle closure glaucoma or pigment dispersion syndrome.
Traditionally, chemotherapeutic adjuvants, such as mitomycin C (MMC) or 5-fluorouracil (5-FU), are applied with soaked sponges on the wound bed to prevent filtering blebs from scarring by inhibiting fibroblast proliferation.
Contemporary alternatives to prevent the scarring of the meshwork opening include the sole or combinative implementation of nonchemotherapeutic adjuvants such as the Ologen collagen matrix, which has been clinically shown to increase the success rates of surgical treatment.
These are indicated for glaucoma patients not responding to maximal medical therapy, with previous failed guarded filtering surgery (trabeculectomy).
[citation needed] In order to prevent wound adhesion after deep scleral excision and to maintain good filtering results, NPDS as with other non-penetrating procedures is sometimes performed with a variety of biocompatible spacers or devices, such as the Aquaflow collagen wick,[95] ologen Collagen Matrix,[84][96][97] or Xenoplast glaucoma implant.
For people with chronic closed-angle glaucoma, lens extraction can relieve the block created by the pupil and help regulate the intraocular pressure.
A review of people with primary open-angle glaucoma and ocular hypertension concluded that medical IOP-lowering treatment slowed down the progression of visual field loss.
[105] The association of elevated intraocular pressure (IOP) and glaucoma was first described by Englishman Richard Banister in 1622: "...that the Eye be grown more solid and hard, then naturally it should be...".
[107] The invention of the ophthalmoscope by Hermann Helmholtz in 1851 enabled ophthalmologists for the first time to identify the pathological hallmark of glaucoma, the excavation of the optic nerve head due to retinal ganglion cell loss.
About half a century later, Hans Goldmann in Bern, Switzerland, developed his applanation tonometer which still today - despite numerous new innovations in diagnostics - is considered the gold standard of determining this crucial pathogenic factor.
[108] The first drug to reduce IOP, pilocarpine, was introduced in the 1870s; other major innovations in pharmacological glaucoma therapy were the introduction of beta blocker eye drops in the 1970s and of prostaglandin analogues and topical (locally administered) carbonic anhydrase inhibitors in the mid-1990s.
Early surgical techniques like iridectomy and fistulating methods have recently been supplemented by less invasive procedures like small implants, a range of options now widely called MIGS (micro-invasive glaucoma surgery).
The word "glaucoma" comes from the Ancient Greek γλαύκωμα,[109] a derivative of γλαυκός (glaukos),[110] which commonly described the color of eyes which were not dark (i.e. blue, green, light gray).
The TAGS randomised controlled trial investigated if eye drops or trabeculectomy is more effective in treating advanced primary open-angle glaucoma.
[114][115] The LiGHT trial compared the effectiveness of eye drops and selective laser trabeculoplasty for open angle glaucoma.
[116] A 2013 Cochrane systematic review compared the effect of brimonidine and timolol in slowing the progression of open angle glaucoma in adult participants.
Results from a meta-analysis of 33,428 primary open-angle glaucoma (POAG) participants published in 2021 suggest that there are substantial ethnic and racial disparities in clinical trials in the US.