Imatinib
Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral targeted therapy medication used to treat cancer.
Severe side effects may include fluid retention, gastrointestinal bleeding, bone marrow suppression, liver problems, and heart failure.
The U.S. Food and Drug Administration (FDA) has approved imatinib as first-line treatment for Philadelphia chromosome-positive CML, both in adults and children.
The drug is approved in multiple contexts of Philadelphia chromosome-positive CML, including after stem cell transplant, in blast crisis, and newly diagnosed.
[12] Due in part to the development of imatinib and related drugs, the five-year survival rate for people with chronic myeloid leukemia increased from 31% in 1993, to 59% in 2009,[13] to 70% in 2016.
The FDA has approved imatinib for use in adults with relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), myelodysplastic/myeloproliferative diseases associated with platelet-derived growth factor receptor gene rearrangements, aggressive systemic mastocytosis without or an unknown D816V c-KIT mutation, hypereosinophilic syndrome and/or chronic eosinophilic leukemia who have the FIP1L1-PDGFRα fusion kinase (CHIC2 allele deletion) or FIP1L1-PDGFRα fusion kinase negative or unknown, unresectable, recurrent and/or metastatic dermatofibrosarcoma protuberans.
[18] For treatment of progressive plexiform neurofibromas associated with neurofibromatosis type I, early research has shown potential for using the c-KIT tyrosine kinase blocking properties of imatinib.
[25] Cautions include:[26] The most common side effects include nausea, vomiting, diarrhea, headaches, leg aches/cramps, fluid retention, visual disturbances, itchy rash, lowered resistance to infection, bruising or bleeding, loss of appetite,[27] weight gain, reduced number of blood cells (neutropenia, thrombocytopenia, anemia), and edema.
[28] In some individuals, imatinib use was reported to be associated with left ventricular dysfunction which sometimes progressed to congestive cardiac failure despite an absence of prior heart disease.
Its use is advised against in people on strong CYP3A4 inhibitors such as clarithromycin, chloramphenicol, ketoconazole, ritonavir and nefazodone due to its reliance on CYP3A4 for metabolism.
Imatinib also acts as an inhibitor of CYP3A4, 2C9 and 2D6, increasing the plasma concentrations of a number of other drugs like simvastatin, ciclosporin, pimozide, warfarin, metoprolol, and possibly paracetamol.
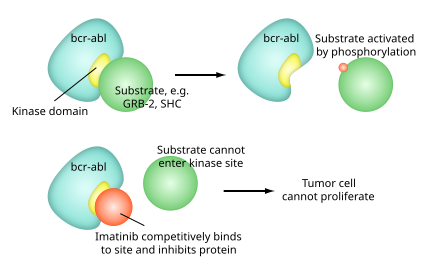
Imatinib is specific for the TK domain in abl (the Abelson proto-oncogene), c-kit and PDGF-R (platelet-derived growth factor receptor).
In chronic myelogenous leukemia, the Philadelphia chromosome leads to a fusion protein of abl with bcr (breakpoint cluster region), termed bcr-abl.
[37] Imatinib is quite selective for bcr-abl, though it does also inhibit other targets mentioned above (c-kit and PDGF-R), as well as ABL2 (ARG) and DDR1 tyrosine kinases and NQO2 – an oxidoreductase.
[39] Inhibition of the bcr-abl tyrosine kinase also stimulates its entry in to the nucleus, where it is unable to perform any of its normal anti-apoptopic functions, leading to tumor cell death.
Metabolism of imatinib occurs in the liver and is mediated by several isozymes of the cytochrome P450 system, including CYP3A4 and, to a lesser extent, CYP1A2, CYP2D6, CYP2C9, and CYP2C19.
It blocks the activity of Abelson cytoplasmic tyrosine kinase (ABL), c-Kit and the platelet-derived growth factor receptor (PDGFR).
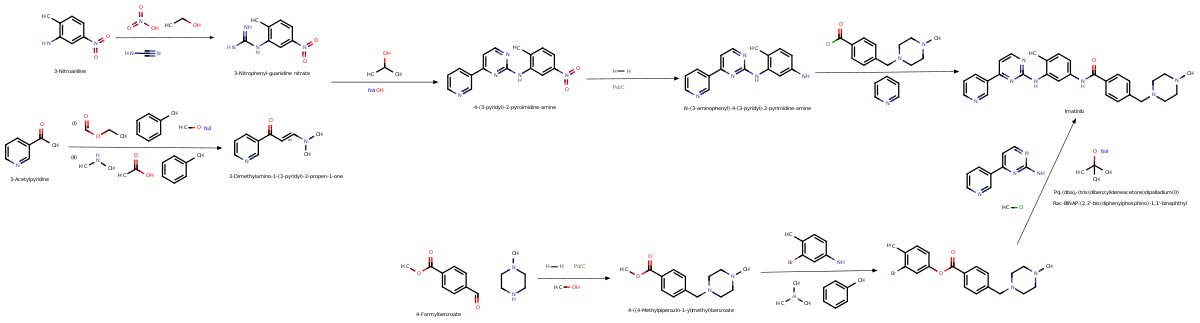
[42] Imatinib was invented in the late 1990s by scientists at Ciba-Geigy (which merged with Sandoz in 1996 to become Novartis), in a team led by the British biochemist Nicholas Lydon and that included Elisabeth Buchdunger and Jürg Zimmermann,[43] and its use to treat CML was driven by oncologist Brian Druker of Oregon Health & Science University (OHSU).
[44] Other major contributions to imatinib development were made by biologist Anthony R. Hunter at Salk Institute for Biological Studies in La Jolla, California, Carlo Gambacorti-Passerini, a physician, scientist, and hematologist at the University of Milano Bicocca, Italy, John Goldman at Hammersmith Hospital in London, and later on by Charles Sawyers of Memorial Sloan Kettering Cancer Center in New York.
This lead compound was then tested and modified by the introduction of methyl and benzamide groups to give it enhanced binding properties, resulting in imatinib.
Druker, Lydon and Sawyers received the Lasker-DeBakey Clinical Medical Research Award in 2009 for "converting a fatal cancer into a manageable chronic condition".
[72] By 2016, the average wholesale price had increased to $120,000 (equivalent to $152,346 in 2023) a year, according to an analysis prepared for The Washington Post by Stacie Dusetzina of the University of North Carolina at Chapel Hill.
[76] Novartis fought a seven-year, controversial battle to patent Gleevec in India, and took the case all the way to the Indian Supreme Court.
These changes came into effect in 2005, so Novartis' patent application waited in a "mailbox" with others until then, under procedures that India instituted to manage the transition.
[83][84] When examination of Novartis' patent application began in 2005, it came under immediate attack from oppositions initiated by generic companies that were already selling Gleevec in India and by advocacy groups.
It was shown to reduce both the smooth muscle hypertrophy and hyperplasia of the pulmonary vasculature in a variety of disease processes, including portopulmonary hypertension.
[89] However, a long-term trial of Imatinib in people with pulmonary arterial hypertension was unsuccessful, and serious and unexpected adverse events were frequent.
In laboratory settings, imatinib is being used as an experimental agent to suppress platelet-derived growth factor (PDGF) by inhibiting its receptor (PDGF-Rβ).
[94] Another study suggests that imatinib may not need to cross the blood–brain barrier to be effective at treating Alzheimer's, as the research indicates the production of beta-amyloid may begin in the liver.
[96] A formulation of imatinib with a cyclodextrin (Captisol) as a carrier to overcome the blood–brain barrier has shown reversal of opioid tolerance in a 2012 study in rats.