Phenoxy herbicide
[2] Analogues of each of these three compounds, with an extra methyl group attached next to the carboxylic acid, were subsequently commercialised as mecoprop, dichlorprop and fenoprop.
[4] All the auxin herbicides retain activity when applied as salts and esters since these are also capable of producing the parent acid in situ.
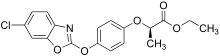
In 1973, Hoechst AG filed patents on a new class of compound, the aryloxphenoxypropionates, which showed such selectivity and led to the commercialisation of diclofop.
Then the Japanese company Ishihara Sangyo Kaisha (ISK) found improved biological activity in an analogue, chlorazifop, which replaced the aryloxy portion of diclofop with a pyridine ring containing the same two chlorine substituents.
[10] This group of herbicides acts by inhibiting plant acetyl-CoA carboxylase (ACCase), a completely different mechanism of action to that of the auxins.
[11][12] Their selectivity for grasses arises because they target the isoform of the enzyme present only in the plastids of these species, making them ineffective on broad-leaf weeds and other organisms including mammals.
Cummins et al., 1999, 2009, and 2013 find that Alopecurus myocuroides's mechanism of fenoxaprop-P-ethyl resistance reduces hydrogen peroxide concentrations at the application site, while the wild type responds with an increase.



Diclofop: X=CH, R 1 =R 2 =Cl
Chlorazifop: X=N, R 1 =R 2 =Cl
Fluazifop: X=N, R 1 =CF 3 , R 2 =H
Haloxyfop: X=N, R 1 =CF 3 , R 2 =Cl