Helicobacter pylori
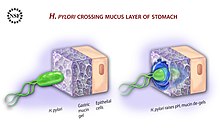
[1][2] Its helical body (from which the genus name Helicobacter derives) is thought to have evolved to penetrate the mucous lining of the stomach, helped by its flagella, and thereby establish infection.
[16] Some studies suggest that H. pylori plays an important role in the natural stomach ecology by influencing the type of bacteria that colonize the gastrointestinal tract.
This analysis of its transcription confirmed the known acid induction of major virulence loci, including the urease (ure) operon and the Cag pathogenicity island (PAI).
[43] An infection with Helicobacter pylori can either have no symptoms even when lasting a lifetime, or can harm the stomach and duodenal linings by inflammatory responses induced by several mechanisms associated with a number of virulence factors.
[47][48] Extragastric complications that have been linked to H. pylori include anemia due either to iron-deficiency or vitamin B12 deficiency, diabetes mellitus, cardiovascular, and certain neurological disorders.
[16] Peptic ulcers are a consequence of inflammation that allows stomach acid and the digestive enzyme pepsin to overwhelm the protective mechanisms of the mucous membranes.
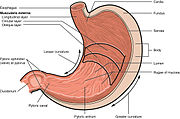
[49] In people producing large amounts of acid, H. pylori colonizes near the pyloric antrum (exit to the duodenum) to avoid the acid-secreting parietal cells at the fundus (near the entrance to the stomach).
The many virulence factors of H. pylori include its flagella, the production of urease, adhesins, serine protease HtrA (high temperature requirement A), and the major exotoxins CagA and VacA.
[7] H. pylori infection is associated with epigenetically reduced efficiency of the DNA repair machinery, which favors the accumulation of mutations and genomic instability as well as gastric carcinogenesis.
Its expression is not only required for establishing initial colonization in the breakdown of urea to carbonic acid and ammonia, but is also essential for maintaining chronic infection.
It has been proposed that BabA's acid responsiveness enables adherence while also allowing an effective escape from an unfavorable environment such as a low pH that is harmful to the organism.
The low GC-content of the cag PAI relative to the rest of the Helicobacter genome suggests the island was acquired by horizontal transfer from another bacterial species.
[115] Pathogenic strains of H. pylori have been shown to activate the epidermal growth factor receptor (EGFR), a membrane protein with a TK domain.
Activation of the EGFR by H. pylori is associated with altered signal transduction and gene expression in host epithelial cells that may contribute to pathogenesis.
[124] H. pylori has to not only survive the harsh gastric acidity but also the sweeping of mucus by continuous peristalsis, and phagocytic attack accompanied by the release of reactive oxygen species.
The AddAB helicase-nuclease complex resects DSBs and loads RecA onto single-strand DNA (ssDNA), which then mediates strand exchange, leading to homologous recombination and repair.
H. pylori mutants that are defective in RuvC have increased sensitivity to DNA-damaging agents and to oxidative stress, exhibit reduced survival within macrophages, and are unable to establish successful infection in a mouse model.
Other indications that prompt testing for H. pylori include long term aspirin or other non-steroidal anti-inflammatory use, unexplained iron deficiency anemia, or in cases of immune thrombocytopenic purpura.
The most accurate method for detecting H. pylori infection is with a histological examination from two sites after endoscopic biopsy, combined with either a rapid urease test or microbial culture.
[46][141][142] Due to H. pylori's role as a major cause of certain diseases (particularly cancers) and its consistently increasing resistance to antibiotic therapy, there is an obvious need for alternative treatments.
Probiotic yogurts containing lactic acid bacteria Bifidobacteria and Lactobacillus exert a suppressive effect on H. pylori infection, and their use has been shown to improve the rates of eradication.
[54][175][177][176] If unresponsive or showing a deterioration, a more conventional chemotherapy (CHOP), immunotherapy, or local radiotherapy can be considered, and any of these or a combination have successfully treated these more advanced types.
[51][185] The hypothesis is not universally accepted, as several randomized controlled trials failed to demonstrate worsening of acid reflux disease symptoms following eradication of H. pylori.
[17] He postulates that the changes in gastric physiology caused by the loss of H. pylori account for the recent increase in incidence of several diseases, including type 2 diabetes, obesity, and asthma.
[196] Before the research of Marshall and Warren, German scientists found spiral-shaped bacteria in the lining of the human stomach in 1875, but they were unable to culture them, and the results were eventually forgotten.
After unsuccessful attempts at culturing the bacteria from the stomach, they finally succeeded in visualizing colonies in 1982, when they unintentionally left their Petri dishes incubating for five days over the Easter weekend.
In their original paper, Warren and Marshall contended that most stomach ulcers and gastritis were caused by bacterial infection and not by stress or spicy food, as had been assumed before.
In 1994, the National Institutes of Health stated most recurrent duodenal and gastric ulcers were caused by H. pylori, and recommended antibiotics be included in the treatment regimen.
[208] The Group is involved with the Annual International Workshop on Helicobacter and Related Bacteria,[209] (renamed as the European Helicobacter and Microbiota Study Group[210]), the Maastricht Consensus Reports (European Consensus on the management of H. pylori),[211][212][213][214] and other educational and research projects, including two international long-term projects: Results from in vitro studies suggest that fatty acids, mainly polyunsaturated fatty acids, have a bactericidal effect against H. pylori, but their in vivo effects have not been proven.
[8] The Murdoch Children's Research Institute is working at developing a vaccine that instead of specifically targeting the bacteria, aims to inhibit the inflammation caused that leads to the associated diseases.