Respiratory syncytial virus
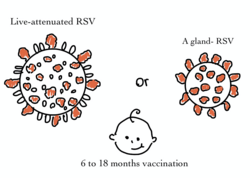
In May 2023, the US Food and Drug Administration (FDA) approved the first RSV vaccines, Arexvy (developed by GSK plc) and Abrysvo (Pfizer).
[20] Childhood RSV infections are fairly self-limited with typical upper respiratory tract signs and symptoms, such as nasal congestion, runny nose, cough, and low-grade fever.
If present, symptoms are generally isolated to the upper respiratory tract: runny nose, sore throat, fever, and malaise.
"[28] Expedient and proper medical care is important for older adults as waiting or receiving a misdiagnosis can be associated with an increased risk of complications.
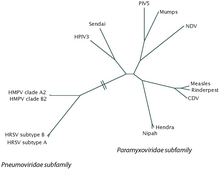
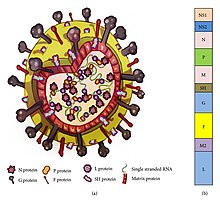
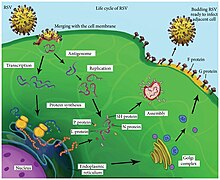
[2] Its name comes from the fact that F proteins on the surface of the virus cause neighboring cell membranes to merge, creating large multinucleated syncytia.
[4] After binding to its target on the host cell surface (its exact ligand remains unclear), PreF undergoes a conformational change during which Ø is lost.
This complementary strand is used as a template to construct genomic negative-sense RNA, which is packaged into nucleocapsids and transported to the plasma membrane for assembly and particle budding.
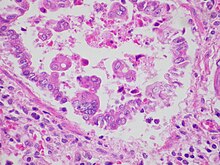
[2] Infection causes generalized inflammation within the lungs, including the migration and infiltration of inflammatory cells (such as monocytes and T-cells), epithelial necrosis, edema, and increased mucous production.
[2][3] After recovery of "respiratory diseases associated with RSV infection, the virus interferes with the establishment of immunological memory, which leads to recurrent reinfections.
[42] Specifically, the irregular curl and low bond energy of the G protein make it prone to conformational changes, affecting its immunogenicity and potentially modulating the immune response.
Evidence suggests that RSV glycoprotein G plays a crucial role in immune modulation during infection, affecting cytokine expression and the antiviral response.
[42] In addition, positive selection pressure drives the dominance of certain genotypes over others, potentially driven by mutations within specific regions of the G gene.
This multifaceted immunomodulatory arsenal likely contributes to RSV's ability to cause mild respiratory symptoms in most cases, yet it poses a severe threat to vulnerable populations such as infants and the elderly, potentially leading to life-threatening lung disease characterized by immune dysregulation.
RSV has evolved numerous strategies to evade the host's antiviral response, with over half of its proteins exerting immunomodulatory effects.
[citation needed] Molecular assays, such as nucleic acid amplification tests (NAATs), enable sensitive detection of very small amounts of virus in nasopharyngeal swabs and aspirates.
[citation needed] In traditional viral culture, a sample of the virus is introduced to different cell lines and allowed to replicate so it can be studied.
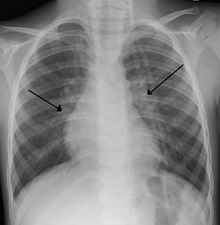
[2] Chest X-ray findings in children with RSV bronchiolitis are generally nonspecific and include perihilar markings, patchy hyperinflation, and atelectasis.
[20] However, the American Academy of Pediatrics (AAP) does not recommend routine imaging for children with presumed RSV bronchiolitis because it does not change clinical outcomes and is associated with increased antibiotic use.
[5] In adults with RSV infection, chest films are often normal or demonstrate nonspecific changes consistent with viral pneumonia, such as patchy bilateral infiltrates.
The introduction of antivirals and vaccines, coupled with advanced diagnostic techniques, holds promise for reducing RSV's global impact in the coming years.
[46][3] The primary pharmaceutical developers, GSK and Pfizer, obtained Food and Drug Administration (FDA) approval for RSV vaccines targeting adults aged 60 and above.
[49] Addressing the more challenging aspect, the need for a newborn vaccine, researchers employed a pregnancy-administered approach to protect infants during the first six months, a critical period for RSV susceptibility.
While unanimous in favor of efficacy, the committee voted 10 to 4 for safety, with concerns about a slightly higher premature birth rate in the vaccinated group.
[50][51][52] Historically, RSV-specific intravenous immunoglobin (IVIG) was used to provide passive immunity to prevent RSV infection and hospitalization in the highest-risk infants.
[56] Nirsevimab requires only one dose that lasts the entire RSV season, unlike palivizumab, which has to be injected about once a month for up to four times to remain effective.
[53] Beyond vaccines, AstraZeneca and Sanofi introduced nirsevimab, a prophylactic monoclonal antibody with 75% efficacy against RSV cases in infants under one year.
A 2024 JAMA Open article suggested a rise in sudden unexpected infant deaths (SUID) may be connected to an unusual surge of RSV in 2021.
[72] The study revealed that the risk of SUID was highest from June to December 2021, coinciding with an off-season spike in RSV hospitalizations after the virus deviated from its typical winter pattern in 2020.
However, it is now recognized as a significant cause of morbidity and mortality in certain adult populations, including the elderly and those with underlying heart or lung diseases.
[75] The Children's Hospital Association and the American Academy of Pediatrics asked US President Joe Biden to declare a state of emergency.