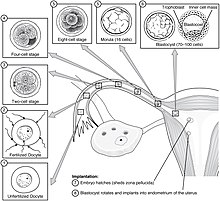
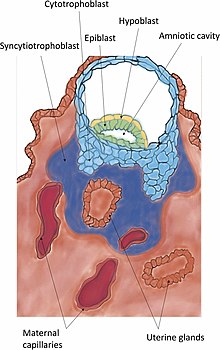
Implantation (embryology)
Implantation, also known as nidation,[1] is the stage in the mammalian embryonic development in which the blastocyst hatches, attaches, adheres, and invades into the endometrium of the female's uterus.
[3] An implanted embryo is detected by the presence of increased levels of human chorionic gonadotropin (hCG) in a pregnancy test.
[4] The endocannabinoid system plays a vital role in this synchrony in the uterus, influencing uterine receptivity, and embryo implantation.
[5] The embryo expresses cannabinoid receptors early in its development that are responsive to anandamide (AEA) secreted in the uterus.
[13] During this migration the zygote undergoes a number of cell divisions that creates a ball of 16 compacted blastomeres called a morula.
Mechanisms in the latter are indicated by the fact that the zona pellucida remains intact if an unfertilized egg is placed in the uterus under the same conditions.
[17] The blastocyst also produces cytokines, both pro-inflammatory and anti-inflammatory, that have crucial roles during implantation and other stages of pregnancy.
Cytokines are also present in the uterine milk which might regulate the development and function of the blastocyst but there is no evidence to support their involvement in hatching.
The trophoblast have binding fiber connections, laminin, collagen type IV, and integrins that assist in this adhesion process.
[22][23] An in vitro model of implantation gave evidence to support the hypothesis that L-selectin mediates apposition of the blastocyst to the uterine epithelium by interacting with its ligands.
[28][29] When the syncytiotrophoblast reaches the basal membrane beneath the decidual cells, it dislodges them to further invade into the uterine stroma.
Degradation is achieved by the secretion of tumor necrosis factor-alpha from the syncytiotrophoblast, which inhibits the expression of CAMs and beta-catenin.
These cells remodel the spiral arteries to improve and secure maternal blood flow to the growing embryo.
There is also evidence that this process occurs with the uterine veins, stabilizing them to improve drainage of fetal blood and metabolic wastes.
[32] Pre-implantation blastocysts have been shown to be capable of secreting growth factors, hormones and trypsin-like proteases to participate in the hatching process.
[36] The synchrony gives a short period of receptivity known as the window of implantation, and involves much crosstalk between the blastocyst and the endometrium at this stage.
[37][38][39] The endocannabinoid system plays a vital role in this synchrony in the uterus, influencing uterine receptivity, and embryo implantation.
[5] The embryo expresses cannabinoid receptors early in its development that are responsive to anandamide (AEA) secreted in the uterus.
[5][40] IL-6 and FAAH are both crucial for uterine receptivity and together with AEA there is seen to be a link with adequate endometrial thickness that sustains pregnancy.
This, in turn, dislodges the decidual cells from their connection to the underlying basal lamina, which enables the blastocyst to perform the succeeding invasion.
[41] In humans uterine receptivity is optimum on days 20-24 of the secretory phase of the menstrual cycle when luteinizing hormone levels are at their peak.
[41] Pinopodes are formed by the swelling of these epithelial cells, and the fusing together of a number of microvilli, to reach a maximum size.
[citation needed] The endometrium increases thickness, becomes vascularized and its glands grow to be tortuous and boosted in their secretions.
[citation needed] Furthermore, the surface of the endometrium produces a kind of rounded cells, which cover the whole area toward the uterine cavity.
In that time, it cannot receive nourishment directly from the blood of the mother, and must rely on secreted nutrients into the uterine cavity, e.g. iron and fat-soluble vitamins.
[48] Implantation failure is considered to be caused by inadequate uterine receptivity in two-thirds of cases, and by problems with the embryo itself in the other third.
[50] As part of the organ-on-a-chip program, an endometrium-on-a-chip has been developed to model the functioning of the endometrium that could more clearly identify causes of implantation failure.
[52] In women with more than three implantation failures in assisted reproduction, a review of several small randomized controlled studies estimated that the use of adjunct low molecular weight heparin improves live birth rate by approximately 80%.
[53] Luteal phase support can include the use of progesterone and human chorionic gonadotropin (hCG) to improve the chances of a successful implantation.
[55] Bleeding and spotting are common during the luteal phase of the menstrual cycle, and early stages of pregnancy, but are unrelated to implantation.