Intervalence charge transfer
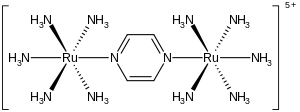
In chemistry, intervalence charge transfer, often abbreviated IVCT or even IT, is a type of charge-transfer band that is associated with mixed valence compounds.
The term is frequently extended to the case of metal-to-metal charge transfer between non-equivalent metal centres.
The band is usually found in the visible or near infrared region of the spectrum and is broad.
Since the energy states of valence tautomers affect the IVCT band, the strength of electronic interaction between the sites, known as α (the mixing coefficient), can be determined by analysis of the IVCT band.
[2] Depending on the value of α, mixed valence complexes are classified into three groups: