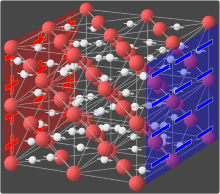
Ionic conductivity (solid state)
In some solids, selected ions are highly mobile allowing ionic conduction.
A well-known ion conductive solid is β''-alumina ("BASE"), a form of aluminium oxide that has channels through which sodium cations can hop.
Michael Faraday established in 1839 that the laws of electrolysis are also obeyed in ionic solids like lead(II) fluoride (PbF2) and silver sulfide (Ag2S).
[clarification needed] This high temperature phase of AgI is an example of a superionic conductor.
The present record holder for ionic conductivity is the related material Ag2[HgI4].