Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction.
Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell.
Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure elements, precedes the coinage of the term and formal description by Faraday.
[2] In 1785 a Dutch scientist named Martin van Marum created an electrostatic generator that he used to reduce tin, zinc and antimony from their salts using a process later known as electrolysis.
During preliminary experiments, Humphry Davy hypothesized that when two elements combine to form a compound, electrical energy is released.
[10] On June 26, 1886, Ferdinand Frederick Henri Moissan finally felt comfortable performing electrolysis on anhydrous hydrogen fluoride to create a gaseous fluorine pure element.
[11] While trying to find elemental fluorine through electrolysis of fluoride salts, many chemists perished including Paulin Louyet and Jérôme Nicklès.
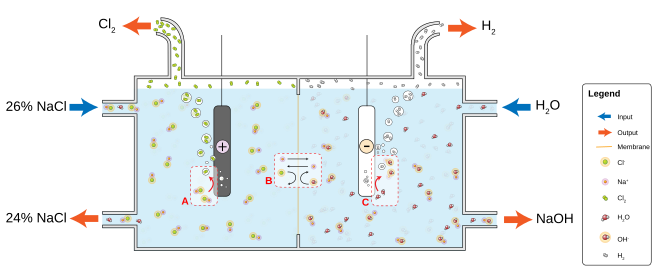
[16][17][18] Electrolysis is the passing of a direct electric current through an electrolyte which is producing chemical reactions at the electrodes and decomposition of the materials.
A partition (e.g. an ion-exchange membrane or a salt bridge) is optional to keep the products from diffusing to the vicinity of the opposite electrode.
The electrolyte is a chemical substance which contains free ions and carries electric current (e.g. an ion-conducting polymer, solution, or a ionic liquid compound).
A direct current supplied by the power source drives the reaction causing ions in the electrolyte to be attracted toward the respective oppositely charged electrode.
Historically, when non-reactive anodes were desired for electrolysis, graphite (called plumbago in Faraday's time) or platinum were chosen.
Platinum erodes very slowly compared to other materials, and graphite crumbles and can produce carbon dioxide in aqueous solutions but otherwise does not participate in the reaction.
The key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons due to the applied potential.
Applying additional voltage, referred to as overpotential, can increase the rate of reaction and is often needed above the thermodynamic value.
In some cases, for instance, in the electrolysis of steam into hydrogen and oxygen at high temperature, the opposite is true and heat energy is absorbed.
[27] Galvanic cells and batteries use spontaneous, energy-releasing redox reactions to generate an electrical potential that provides useful power.
In principle, the voltage required to electrolyze a salt solution can be derived from the standard electrode potential for the reactions at the anode and cathode.
In terms of electrolysis, this table should be interpreted as follows: Using the Nernst equation the electrode potential can be calculated for a specific concentration of ions, temperature and the number of electrons involved.
For pure water (pH 7): Comparable figures calculated in a similar way, for 1 M zinc bromide, ZnBr2, are −0.76 V for the reduction to Zn metal and +1.10 V for the oxidation producing bromine.
The efficiency of an electrolyser is a measure of the enthalpy contained in the hydrogen (to undergo combustion with oxygen or some other later reaction), compared with the input electrical energy.
Note that fuel cells (not electrolysers) cannot use this full amount of heat/enthalpy, which has led to some confusion when calculating efficiency values for both types of technology.
Hydrogen is used for the creation of ammonia for fertilizer via the Haber process, and converting heavy petroleum sources to lighter fractions via hydrocracking.
[45][46] The carbon/hydrocarbon assisted water electrolysis (so-called CAWE) process for hydrogen generation would perform this operation in a single electrochemical reactor.
[47] This process utilizes solid coal/carbon particles or powder as fuels dispersed in acid/alkaline electrolyte in the form of slurry and the carbon contained source co-assist in the electrolysis process as following theoretical overall reactions:[48] or Thus, this CAWE approach is that the actual cell overpotential can be significantly reduced to below 1.0 V as compared to 1.5 V for conventional water electrolysis.
A specialized application of electrolysis involves the growth of conductive crystals on one of the electrodes from oxidized or reduced species that are generated in situ.
The technique has been used to obtain single crystals of low-dimensional electrical conductors, such as charge-transfer salts and linear chain compounds.
[49][50] The current method of producing steel from iron ore is very carbon intensive, in part to the direct release of CO2 in the blast furnace.
While these methods are promising, they struggle to be cost competitive because of the large economies of scale keeping the price of blast furnace iron low.
The inclusion of magnesium and calcium ions in the seawater makes the production of alkali hydroxides possible that could form scales in the electrolyser cell, cutting down on lifespan and increasing the need for maintenance.