Ion exchange
Ion exchange usually describes a process of purification of aqueous solutions using solid polymeric ion-exchange resin.
[1] Aside from its use to purify drinking water, the technique is widely applied for purification and separation of a variety of industrially and medicinally important chemicals.
Typical ion exchangers are ion-exchange resins (functionalized porous or gel polymer), zeolites, montmorillonite, clay, and soil humus.
However, the simultaneous exchange of cations and anions is often performed in mixed beds, which contain a mixture of anion- and cation-exchange resins, or passing the solution through several different ion-exchange materials.
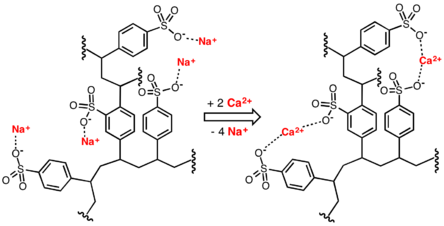
The resin is composed of cross-linked organic polymers, typically polystyrene matrix and functional groups where the ion exchange process takes place.
In domestic filtration systems ion exchange is one of the alternatives for water softening in households along with reverse osmosis (RO) membranes.
Compared to RO membranes, ion exchange requires repetitive regeneration when inlet water is hard (has high mineral content).
[citation needed] There are two series of rare-earth metals, the lanthanides and the actinides, both of whose families all have very similar chemical and physical properties.
The ion-exchange process is also used to separate other sets of very similar chemical elements, such as zirconium and hafnium, which is also very important for the nuclear industry.
Ion-exchange resins in the form of thin membranes are also used in chloralkali process, fuel cells, and vanadium redox batteries.
Neutralized deionizer regeneration wastewater contains all of the removed ions plus 2.5–5 times their equivalent concentration as sodium sulfate.