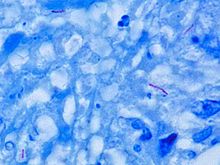
Mycobacterium tuberculosis
[1][2] First discovered in 1882 by Robert Koch, M. tuberculosis has an unusual, waxy coating on its cell surface primarily due to the presence of mycolic acid.
This coating makes the cells impervious to Gram staining, and as a result, M. tuberculosis can appear weakly Gram-positive.
The most frequently used diagnostic methods for tuberculosis are the tuberculin skin test, acid-fast stain, culture, and polymerase chain reaction.
[8] M. tuberculosis is characterized in tissue by caseating granulomas containing Langhans giant cells, which have a "horseshoe" pattern of nuclei.
Compared to other commonly studied bacteria, M. tuberculosis has a remarkably slow growth rate, doubling roughly once per day.
However, major spread is through air droplets originating from a person who has the disease either coughing, sneezing, speaking, or singing.
[18][10] Specifically, M. tuberculosis blocks the bridging molecule, early endosomal autoantigen 1 (EEA1); however, this blockade does not prevent fusion of vesicles filled with nutrients.
[20] More recently, M. tuberculosis has been shown to secrete and cover itself in 1-tuberculosinyladenosine (1-TbAd), a special nucleoside that acts as an antacid, allowing it to neutralize pH and induce swelling in lysosomes.
[23] As a result of having apoptosis being suppressed, it provides M. tuberculosis with a safe replicative niche, and so the bacteria are able to maintain a latent state for a prolonged time.
[25][26][27][28][29] The ability to construct M. tuberculosis mutants and test individual gene products for specific functions has significantly advanced the understanding of its pathogenesis and virulence factors.
In addition, pre-existing first-line TB drugs such as rifampicin and streptomycin have decreased efficiency in clearing intracellular M. tuberculosis due to their inability to effectively penetrate the macrophage niche.
[33] Since PPM1A levels are elevated during M. tuberculosis infections, by inhibiting the PPM1A signalling pathways, it could potentially be a therapeutic method to kill M. tuberculosis-infected macrophages by restoring its normal apoptotic function in defence of pathogens.
[23] The ability to restore macrophage apoptosis to M. tuberculosis-infected ones could improve the current tuberculosis chemotherapy treatment, as TB drugs can gain better access to the bacteria in the niche.
[36] This has now been superseded by variable numbers of tandem repeats (VNTR), which is technically easier to perform and allows better discrimination between strains.
[42] M. tuberculosis is considered to be multidrug-resistant (MDR TB) if it has developed drug resistance to both rifampicin and isoniazid, which are the most important antibiotics used in treatment.
Additionally, extensively drug-resistant M. tuberculosis (XDR TB) is characterized by resistance to both isoniazid and rifampin, plus any fluoroquinolone and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin).
Bacteria isolated from the lungs of infected mice were shown to preferentially use fatty acids over carbohydrate substrates.
[54] Due to the influence of methylation on gene expression at some locations in the genome,[52] it has been hypothesized that IMM may give rise to phenotypic diversity, and partially responsible for the global success of Beijing sublineage.
[58][59] In addition to M. tuberculosis, the MTBC has a number of members infecting various animal species, including M. africanum, M. bovis (Dassie's bacillus), M. caprae, M. microti, M. mungi, M. orygis, and M. pinnipedii.
By calibrating the mutation rate of M. tuberculosis to match this narrative, the study suggested that MTBC evolved 40,000–70,000 years ago.
Despite an additionally slow evolution rate, the emergence and spread of antibiotic resistance in M. tuberculosis poses an increasing threat to global public health.
Countries facing the highest rates of ABR TB China, India, Russia, and South Africa.
Isoniazid function occurs through the inhibition of mycolic acid synthesis through the NADH-dependent enoyl-acyl carrier protein (ACP)-reductase.
[citation needed] An intrinsic contributor to the antibiotic resistant nature of M. tuberculosis is its unique cell wall.
[82] Due to its essential nature, MmpL3 inhibitors show promise as alternative therapeutic measures in the age of antibiotic resistance.
Inhibition of MmpL3 function showed an inability to transport trehalose monomycolate - an essential cell wall lipid - across the plasma membrane.
Additionally, the ability of many MmpL3 inhibitors to work synergistically with other antitubercular drugs presents a ray of hope in combatting the TB crisis.
[citation needed] As an intracellular pathogen, M. tuberculosis is exposed to a variety of DNA-damaging assaults, primarily from host-generated antimicrobial toxic radicals.
[93] Because of this, it is primarily used in high tuberculosis incidence regions, and is not a recommended vaccine in the United States due to the low risk of infection.
To receive this vaccine in the United States, an individual is required to go through a consultation process with an expert in M. tuberculosis and is only given to those who meet the specific criteria.


- Negative control
- M. tuberculosis : Dry-appearing colonies
- Mycobacterium avium complex : Wet-appearing colonies
- M. gordonae : Yellowish colonies