Light-emitting diode physics
Since these materials have a high index of refraction,[note 1] design features of the devices such as special optical coatings and die shape are required to efficiently emit light.
A LED is a long-lived light source, but certain mechanisms can cause slow loss of efficiency of the device or sudden failure.
Light-emitting diodes are widely used in indicator and display functions, and white LEDs are displacing other technologies for general illumination purposes.
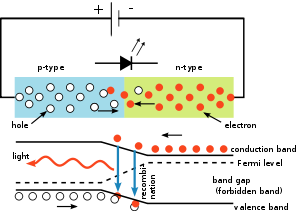
The p–n junction in any direct band gap material emits light when electric current flows through it.
As indirect band gap materials the electrons dissipate energy in the form of heat within the crystalline silicon and germanium diodes, but in gallium arsenide phosphide (GaAsP) and gallium phosphide (GaP) semiconductors, the electrons dissipate energy by emitting photons.
The wavelength of the light emitted, and thus its color, depends on the band gap energy of the materials forming the p-n junction.
In silicon or germanium diodes, the electrons and holes usually recombine by a non-radiative transition, which produces no optical emission, because these are indirect band gap materials.
The materials used for the LED have a direct band gap with energies corresponding to near-infrared, visible, or near-ultraviolet light.
Advances in materials science have enabled making devices with ever-shorter wavelengths, emitting light in a variety of colors.
[1] Internal reflections can escape through other crystalline faces if the incidence angle is low enough and the crystal is sufficiently transparent to not re-absorb the photon emission.
All light rays emanating from the center would be perpendicular to the entire surface of the sphere, resulting in no internal reflections.
Since LEDs installed in real fixtures operate at higher temperature and with driver losses, real-world efficiencies are much lower.
Scientists proved the opposite is true: though the life of an LED is shortened, the efficiency droop is less severe at elevated temperatures.
Typical lifetimes quoted are 25,000 to 100,000 hours, but heat and current settings can extend or shorten this time significantly.
[17] It is important to note that these projections are based on a standard test that may not accelerate all the potential mechanisms that can induce failures in LEDs.
At these temperatures, relatively little heat is lost by radiation, which means that the light beam generated by an LED is cool.
Since the maximum operating temperature of the LED is limited, the thermal resistances of the package, the heat sink and the interface must be calculated.
Medium-power LEDs are often designed to solder directly to a printed circuit board that contains a thermally conductive metal layer.
[28] Quantum dots (QD) are semiconductor nanocrystals with optical properties that let their emission color be tuned from the visible into the infrared spectrum.
The other is direct electrical excitation first demonstrated by Alivisatos et al.[31] One example of the photo-excitation scheme is a method developed by Michael Bowers, at Vanderbilt University in Nashville, involving coating a blue LED with quantum dots that glow white in response to the blue light from the LED.
[32] Quantum dots are also being considered for use in white light-emitting diodes in liquid crystal display (LCD) televisions.
An applied electric field causes electrons and holes to move into the quantum dot layer and recombine forming an exciton that excites a QD.
The tunability of emission wavelengths and narrow bandwidth is also beneficial as excitation sources for fluorescence imaging.