Lanosterol 14 alpha-demethylase
[5] The cytochrome P450 isoenzymes are a conserved group of proteins that serve as key players in the metabolism of organic substances and the biosynthesis of important steroids, lipids, and vitamins in eukaryotes.
[10] Although the structure of 14α-demethylase may vary substantially from one organism to the next, sequence alignment analysis reveals that there are six regions in the protein that are highly conserved in eukaryotes.
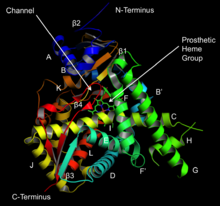
[7] Homology modeling reveals that substrates migrate from the surface of the protein to the enzyme's buried active site through a channel that is formed in part by the A' alpha helix and the β4 loop.
[11][12] Finally, the active site contains a heme prosthetic group in which the iron is tethered to the sulfur atom on a conserved cysteine residue.
[13] During the first two steps, the 14α-methyl group undergoes typical cytochrome monooxygenation in which one oxygen atom is incorporated by the substrate and the other is reduced to water, resulting in the sterol's conversion to a carboxyalcohol and then a carboxyaldehyde.