Lanthanum hydroxide
This produces a gel-like precipitate that can then be dried in air.
[2] Alternatively, it can be produced by hydration reaction (addition of water) to lanthanum oxide.
[3] Lanthanum hydroxide does not react much with alkaline substances, however is slightly soluble in acidic solution.
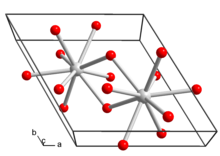
[2] In temperatures above 330 °C it decomposes into lanthanum oxide hydroxide (LaOOH), which upon further heating decomposes into lanthanum oxide (La2O3):[4] Lanthanum hydroxide crystallizes in the hexagonal crystal system.
Each lanthanum ion in the crystal structure is surrounded by nine hydroxide ions in a tricapped trigonal prism.