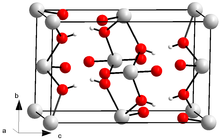
Uranyl hydroxide
Uranyl hydroxide hydrate is precipitated as a colloidal yellowcake from oxidized uranium liquors near neutral pH.
Uranyl hydroxide was once used in glassmaking and ceramics in the colouring of the vitreous phases and the preparation of pigments for high temperature firing.
X-ray diffraction data was gathered and found that this species has expanded interlayer spacing suggesting there may be additional water molecules in between uranyl layers.
This could be due to the strongly basic (OH)− reducing the Lewis acidity of U or because the more complex acetate and nitrate anions provide more degrees of freedom.
[2] A mechanism for oxygen exchange between the UO22+ cations in a highly alkaline solution was proposed and investigated by Shamov et al. in the Journal of the American Chemical Society.