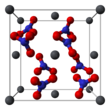
Lead(II) nitrate
It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.
In the nineteenth century lead(II) nitrate began to be produced commercially in Europe and the United States.
Lead(II) nitrate is toxic and must be handled with care to prevent inhalation, ingestion and skin contact.
Due to its hazardous nature, the limited applications of lead(II) nitrate are under constant scrutiny.
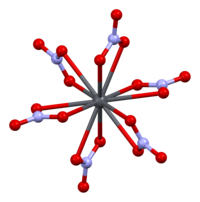
Lead(II) is a hard acceptor; it forms stronger complexes with nitrogen and oxygen electron-donating ligands.
For example, combining lead nitrate and pentaethylene glycol (shortened to EO5 in the referenced paper) in a solution of acetonitrile and methanol followed by slow evaporation produced the compound [Pb(NO3)2EO5].
[19] In the crystal structure for this compound, the EO5 chain is wrapped around the lead ion in an equatorial plane similar to that of a crown ether.
[20] One aspect of this type of complexes is the presence of a physical gap in the coordination sphere; i.e., the ligands are not placed symmetrically around the metal ion.
[26] All inorganic lead compounds are classified by the International Agency for Research on Cancer (IARC) as probably carcinogenic to humans (Category 2A).
[28] Lead is known to substitute for zinc in a number of enzymes, including δ-aminolevulinic acid dehydratase (porphobilinogen synthase) in the haem biosynthetic pathway and pyrimidine-5′-nucleotidase, important for the correct metabolism of DNA and can therefore cause fetal damage.