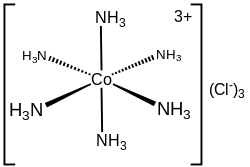
Coordination sphere
In hexamminecobalt(III) chloride ([Co(NH3)6]Cl3), the cobalt cation plus the 6 ammonia ligands comprise the first coordination sphere.
The coordination sphere of this ion thus consists of a central MN6 core "decorated" by 18 N−H bonds that radiate outwards.
Nonetheless the second coordination sphere is relevant to understanding reactions of the metal complex, including the mechanisms of ligand exchange and catalysis.
Such effects can be pronounced in complexes where the ligands in the first coordination sphere are strong hydrogen-bond donors and acceptors, e.g. respectively [Co(NH3)6]3+ and [Fe(CN)6]3−.
[5] Macrocyclic molecules such as cyclodextrins act often as the second coordination sphere for metal complexes.

The NH 3 and Cl groups form a coordination sphere around the central cobalt ion.