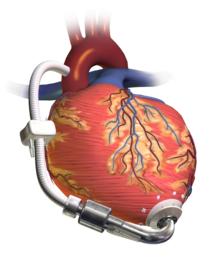
Ventricular assist device
Certain types of VADS may be used in patients with signs of acute (sudden onset) heart failure or cardiogenic shock as a result of an infarction, valvular disease, among other causes.
[9] Extracorporeal Membrane Oxygenation (ECMO) – is a form of mechanical circulatory support typically used in critically ill patients in cardiogenic shock that is established by introducing cannula into the arteries and or veins of the neck, axilla or groin.
Controlled electric currents running through coils contained in the pump housing apply forces to the magnets, which in turn cause the rotors to spin.
Early versions used solid bearings; however, newer pumps, some of which are approved for use in the EU, use either magnetic levitation ("maglev")[19][20][21] or hydrodynamic suspension.
The first left ventricular assist device (LVAD) system was created by Domingo Liotta at Baylor College of Medicine in Houston in 1962.
[23] The first successful long-term implantation of an LVAD was conducted in 1988 by Dr. William F. Bernhard of Boston Children's Hospital Medical Center and Thermedics, Inc. of Woburn, MA, under a National Institutes of Health (NIH) research contract which developed HeartMate, an electronically controlled assist device.
This was funded by a three-year $6.2 million contract to Thermedics and Children's Hospital, Boston, MA, from the National Heart, Lung, and Blood Institute, a program of the NIH.
[24] The early VADs emulated the heart by using a "pulsatile" action where blood is alternately sucked into the pump from the left ventricle then forced out into the aorta.
Since then, he completed a 91-mile charity walk, published two books, lectured widely, hiked in the Swiss Alps and the American West, flew in an ultra-light aircraft, and traveled extensively around the world.
[28][29] Since then, patient Lidia Pluhar has exceeded Houghton's longevity on a VAD, having received a HeartMate II in March 2011 at age 75, and currently continues to use the device.
The smallest device approved by the FDA, the HeartMate II, weighs about 1 pound (0.45 kg) and measures 3 inches (7.6 cm).
[7][8] Several surgical approaches, including interventional decommissioning, off-pump explantation using a custom-made plug and complete LVAD removal through redo sternotomy, have been described with a 5-year survival of up to 80%.
The Harefield Recovery Protocol Study (HARPS) is a clinical trial to evaluate whether advanced heart failure patients requiring VAD support can recover sufficient myocardial function to allow device removal (known as explantation).
HARPS combines an LVAD (the HeartMate XVE) with conventional oral heart failure medications, followed by the novel β2 agonist clenbuterol.
[57] The REMATCH (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) clinical trial began in May 1998 and ran through July 2001 in 20 cardiac transplant centers around the USA.
The trial was designed to compare long-term implantation of left ventricular assist devices with optimal medical management for patients with end-stage heart failure who require, but do not qualify to receive cardiac transplantation.
[58] According to a retrospective cohort study comparing patients treated with a left ventricular assist device versus inotrope therapy while awaiting heart transplantation, the group treated with LVAD had improved clinical and metabolic function at the time of transplant with better blood pressure, sodium, blood urea nitrogen, and creatinine.
[61][62] Due to the use of anticoagulation, bleeding is the most common postoperative early complication after implantation or explantation of VADs, necessitating reoperation in up to 60% of recipients.
[66] Bleeding events may require massive blood transfusions and incur certain risks including infection, pulmonary insufficiency, increased costs, right heart failure, allosensitization, and viral transmission, which can prove fatal or preclude transplantation.
[72] Considering the multitude of risks and lifestyle modifications associated with ventricular assist device implants,[73] it is important for prospective patients to be informed prior to decision making.