Electromagnetic radiation
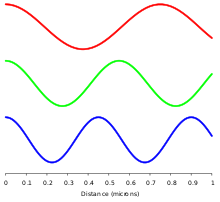
In order of increasing frequency and decreasing wavelength, the electromagnetic spectrum includes: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
[8] Quantum effects provide additional sources of EMR, such as the transition of electrons to lower energy levels in an atom and black-body radiation.
The effect of non-ionizing radiation on chemical systems and living tissue is primarily simply heating, through the combined energy transfer of many photons.
Ionizing radiation can cause chemical reactions and damage living cells beyond simply heating, and can be a health hazard and dangerous.
For example, when electromagnetic radiation is absorbed by matter, particle-like properties will be more obvious when the average number of photons in the cube of the relevant wavelength is much smaller than 1.
[22][23][24] In homogeneous, isotropic media, electromagnetic radiation is a transverse wave,[25] meaning that its oscillations are perpendicular to the direction of energy transfer and travel.
The electric and magnetic parts of the field in an electromagnetic wave stand in a fixed ratio of strengths to satisfy the two Maxwell equations that specify how one is produced from the other.
[28][29][30] An anomaly arose in the late 19th century involving a contradiction between the wave theory of light and measurements of the electromagnetic spectra that were being emitted by thermal radiators known as black bodies.
Planck's theory was based on the idea that black bodies emit light (and other electromagnetic radiation) only as discrete bundles or packets of energy.
[32] Likewise, the momentum p of a photon is also proportional to its frequency and inversely proportional to its wavelength: The source of Einstein's proposal that light was composed of particles (or could act as particles in some circumstances) was an experimental anomaly not explained by the wave theory: the photoelectric effect, in which light striking a metal surface ejected electrons from the surface, causing an electric current to flow across an applied voltage.
Because of the preponderance of evidence in favor of the wave theory, however, Einstein's ideas were met initially with great skepticism among established physicists.
A similar phenomenon occurs for emission, which is seen when an emitting gas glows due to excitation of the atoms from any mechanism, including heat.
[38] Rapidly moving electrons are most sharply accelerated when they encounter a region of force, so they are responsible for producing much of the highest frequency electromagnetic radiation observed in nature.
[41] Herschel used a glass prism to refract light from the Sun and detected invisible rays that caused heating beyond the red part of the spectrum, through an increase in the temperature recorded with a thermometer.
[42] In 1801, German physicist Johann Wilhelm Ritter discovered ultraviolet in an experiment similar to Herschel's, using sunlight and a glass prism.
Maxwell therefore suggested that visible light (as well as invisible infrared and ultraviolet rays by inference) all consisted of propagating disturbances (or radiation) in the electromagnetic field.
After experimenting with high voltages applied to an evacuated tube on 8 November 1895, he noticed a fluorescence on a nearby plate of coated glass.
The origin of the ray differentiates them, gamma rays tend to be natural phenomena originating from the unstable nucleus of an atom and X-rays are electrically generated (and hence man-made) unless they are as a result of bremsstrahlung X-radiation caused by the interaction of fast moving particles (such as beta particles) colliding with certain materials, usually of higher atomic numbers.
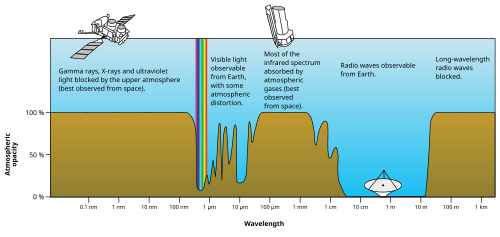
[45]: 308, 9 EM radiation (the designation 'radiation' excludes static electric and magnetic and near fields) is classified by wavelength into radio, microwave, infrared, visible, ultraviolet, X-rays and gamma rays.
Animals that detect infrared make use of small packets of water that change temperature, in an essentially thermal process that involves many photons.
Infrared, microwaves and radio waves are known to damage molecules and biological tissue only by bulk heating, not excitation from single photons of the radiation.
When a photon is absorbed, the retinal permanently changes structure from cis to trans, and requires a protein to convert it back, i.e. reset it to be able to function as a light detector again.
Limited evidence indicate that some reactive oxygen species are created by visible light in skin, and that these may have some role in photoaging, in the same manner as ultraviolet A.
[49] As frequency increases into the ultraviolet, photons now carry enough energy (about three electron volts or more) to excite certain doubly bonded molecules into permanent chemical rearrangement.
This is why ultraviolet at all wavelengths can damage DNA, and is capable of causing cancer, and (for UVB) skin burns (sunburn) that are far worse than would be produced by simple heating (temperature increase) effects.
Electromagnetic-type ionizing radiation extends from the extreme ultraviolet to all higher frequencies and shorter wavelengths, which means that all X-rays and gamma rays qualify.
However, at energies too low to excite water vapor, the atmosphere becomes transparent again, allowing free transmission of most microwave and radio waves.
[53][citation needed] Infrared radiation in the spectral distribution of a black body is usually considered a form of heat, since it has an equivalent temperature and is associated with an entropy change per unit of thermal energy.
There are nontrivial solutions of the homogeneous Maxwell's equations (without charges or currents), describing waves of changing electric and magnetic fields.
This is guaranteed since the generic wave solution is first order in both space and time, and the curl operator on one side of these equations results in first-order spatial derivatives of the wave solution, while the time-derivative on the other side of the equations, which gives the other field, is first-order in time, resulting in the same phase shift for both fields in each mathematical operation.





(1831–1879)

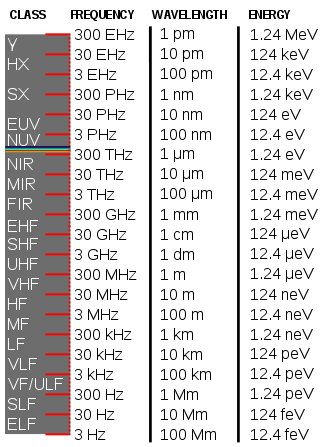
γ = Gamma rays
HX = Hard X-rays
SX = Soft X-Rays
EUV = Extreme- ultraviolet
NUV = Near-ultraviolet
Visible light (colored bands)
NIR = Near- infrared
MIR = Mid-infrared
FIR = Far-infrared
EHF = Extremely high frequency (microwaves)
SHF = Super-high frequency (microwaves)
UHF = Ultrahigh frequency (radio waves)
VHF = Very high frequency (radio)
HF = High frequency (radio)
MF = Medium frequency (radio)
LF = Low frequency (radio)
VLF = Very low frequency (radio)
VF = Voice frequency
ULF = Ultra-low frequency (radio)
SLF = Super-low frequency (radio)
ELF = Extremely low frequency (radio)