Lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants.
[1] Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily.
This finding also suggests that the original function of lignin may have been structural as it plays this role in the red alga Calliarthron, where it supports joints between calcified segments.
An example of composition from an aspen[8] sample is 63.4% carbon, 5.9% hydrogen, 0.7% ash (mineral components), and 30% oxygen (by difference),[9] corresponding approximately to the formula (C31H34O11)n. Lignin is a collection of highly heterogeneous polymers derived from a handful of precursor lignols.
[citation needed] The relative amounts of the precursor "monomers" (lignols or monolignols) vary according to the plant source.
[12] Many grasses have mostly G, while some palms have mainly S.[13] All lignins contain small amounts of incomplete or modified monolignols, and other monomers are prominent in non-woody plants.
[citation needed] Lignin plays a crucial part in conducting water and aqueous nutrients in plant stems.
The polysaccharide components of plant cell walls are highly hydrophilic and thus permeable to water, whereas lignin is more hydrophobic.
[16] Its most commonly noted function is the support through strengthening of wood (mainly composed of xylem cells and lignified sclerenchyma fibres) in vascular plants.
[25] Lignin removed by the kraft process is usually burned for its fuel value, providing energy to power the paper mill.
Two commercial processes exist to remove lignin from black liquor for higher value uses: LignoBoost (Sweden) and LignoForce (Canada).
Higher quality lignin presents the potential to become a renewable source of aromatic compounds for the chemical industry, with an addressable market of more than $130bn.
For example, syringyl (S) lignin is more susceptible to degradation by fungal decay as it has fewer aryl-aryl bonds and a lower redox potential than guaiacyl units.
[31][32] Because it is cross-linked with the other cell wall components, lignin minimizes the accessibility of cellulose and hemicellulose to microbial enzymes, leading to a reduced digestibility of biomass.
Dye-decolorizing peroxidases, or DyPs, exhibit catalytic activity on a wide range of lignin model compounds, but their in vivo substrate is unknown.
[36] Yet, bacterial degradation can be quite extensive,[37] especially in aquatic systems such as lakes, rivers, and streams, where inputs of terrestrial material (e.g. leaf litter) can enter waterways.
[38] In addition to the presence or absence of light, several of environmental factors affect the biodegradability of lignin, including bacterial community composition, mineral associations, and redox state.
[39][40] In shipworms, the lignin it ingests is digested by "Alteromonas-like sub-group" bacteria symbionts in the typhlosole sub-organ of its cecum.
In cooking, lignin in the form of hardwood is an important source of these two compounds, which impart the characteristic aroma and taste to smoked foods such as barbecue.
[46] Thermochemolysis (chemical break down of a substance under vacuum and at high temperature) with tetramethylammonium hydroxide (TMAH) or cupric oxide[47] has also been used to characterize lignins.
[31][32] Increases in the (Ad/Al) value indicate an oxidative cleavage reaction has occurred on the alkyl lignin side chain which has been shown to be a step in the decay of wood by many white-rot and some soft rot fungi.
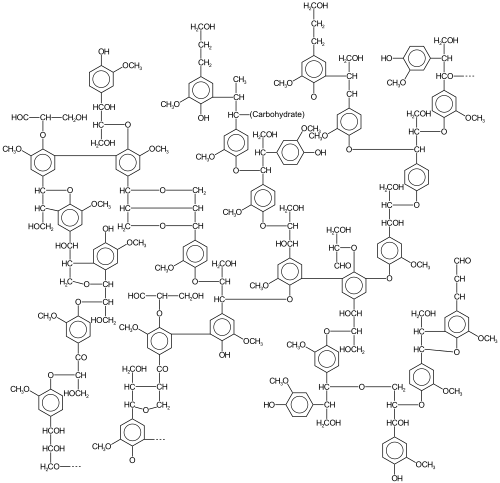

- paracoumaryl alcohol , H
- coniferyl alcohol , G
- sinapyl alcohol , S

