Limnology
[1] The study of limnology includes aspects of the biological, chemical, physical, and geological characteristics of fresh and saline, natural and man-made bodies of water.
While limnology has substantial overlap with freshwater-focused disciplines (e.g., freshwater biology), it also includes the study of inland salt lakes.
Interest in the discipline rapidly expanded, and in 1922 August Thienemann (a German zoologist) and Einar Naumann (a Swedish botanist) co-founded the International Society of Limnology (SIL, from Societas Internationalis Limnologiae).
[10] At the University of Wisconsin-Madison, Edward A. Birge, Chancey Juday, Charles R. Goldman, and Arthur D. Hasler contributed to the development of the Center for Limnology.
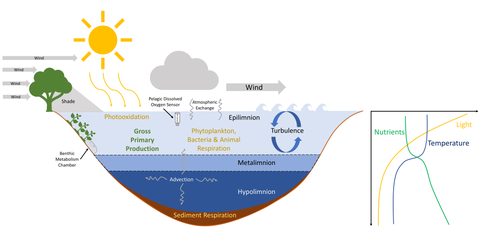
[11][12] Physical properties of aquatic ecosystems are determined by a combination of heat, currents, waves and other seasonal distributions of environmental conditions.
[13] Wetlands vary in size, shape, and pattern however the most common types, marshes, bogs and swamps, often fluctuate between containing shallow, freshwater and being dry depending on the time of year.
The rest of the water column which is deeper and does not receive sufficient amounts of sunlight for plant growth is known as the aphotic zone.
In cold climates, when water cools below 4oC (the temperature of maximum density) many lakes can experience an inverse thermal stratification in winter.
This can be calculated by integrating the area of the lake at each depth interval (Az) multiplied by the difference between the summer (θsz) and winter (θwz) temperatures or
Az(θsz-θwz)[19] The chemical composition of water in aquatic ecosystems is influenced by natural characteristics and processes including precipitation, underlying soil and bedrock in the drainage basin, erosion, evaporation, and sedimentation.
In addition to natural processes, human activities strongly influence the chemical composition of aquatic systems and their water quality.
Physical processes including wind mixing can increase dissolved oxygen concentrations, particularly in surface waters of aquatic ecosystems.
[23] Vertical changes in the concentrations of dissolved oxygen are affected by both wind mixing of surface waters and the balance between photosynthesis and respiration of organic matter.
Nitrogen is generally present as a gas in aquatic ecosystems however most water quality studies tend to focus on nitrate, nitrite and ammonia levels.
[13] Most of these dissolved nitrogen compounds follow a seasonal pattern with greater concentrations in the fall and winter months compared to the spring and summer.
[13] Phosphorus has a different role in aquatic ecosystems as it is a limiting factor in the growth of phytoplankton because of generally low concentrations in the water.
[25] The physical and chemical properties of tropical aquatic environments are different from those in temperate regions, with warmer and more stable temperatures, higher nutrient levels, and more complex ecological interactions.
These scientists largely study the characteristics of inland fresh-water systems such as lakes, rivers, streams, ponds and wetlands.