Lithium cobalt oxide
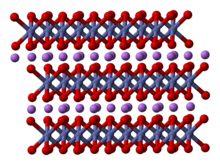
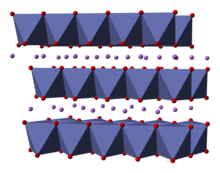
Lithium cobalt oxide is a dark blue or bluish-gray crystalline solid,[4] and is commonly used in the positive electrodes of lithium-ion batteries.
The threefold rotational axis (which is normal to the layers) is termed improper because the triangles of oxygen (being on opposite sides of each octahedron) are anti-aligned.
[11] The compound is now used as the cathode in some rechargeable lithium-ion batteries, with particle sizes ranging from nanometers to micrometers.
[10][9] During charging, the cobalt is partially oxidized to the +4 state, with some lithium ions moving to the electrolyte, resulting in a range of compounds LixCoO2 with 0 < x < 1.
The decomposition of LiCoO2 is a safety concern due to the magnitude of this highly exothermic reaction, which can spread to adjacent cells or ignite nearby combustible material.