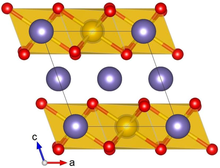
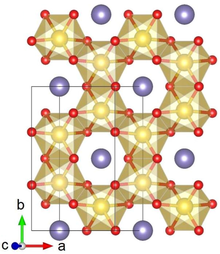
Lithium iridate
It forms black crystals with three slightly different layered atomic structures, α, β, and sometimes γ. Lithium iridate exhibits metal-like, temperature-independent electrical conductivity, and changes its magnetic ordering from paramagnetic to antiferromagnetic upon cooling to 15 K. Li2IrO3 typically crystallizes in the α or β phase, and a rare γ phase has been reported.
[1] Li2IrO3 crystals can be grown by direct sintering of Ir and Li metals, which both oxidize during heating in ambient atmosphere.
The use of Li metal instead of more traditional lithium carbonate, which is easier to handle and store, results in larger crystals.
[1] Lithium iridate is black in color and has a relatively high, temperature-independent electrical conductivity characteristic of metals.
[2] Its both α and β phases exhibit the Kitaev exchange coupling between magnetic spins originating from Ir4+ ions.