Cativa process
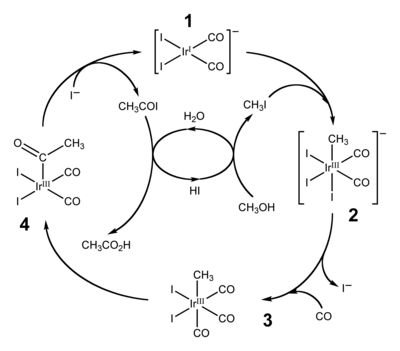
Initial studies by Monsanto had shown iridium to be less active than rhodium for the carbonylation of methanol.
This change reduces the number of drying columns necessary, decreases formation of by-products, such as propionic acid, and suppresses the water gas shift reaction.
This oxidative addition reaction involves the formal insertion of the iridium(I) centre into the carbon-iodine bond of methyl iodide.
After ligand exchange (iodide for carbon monoxide), the migratory insertion of carbon monoxide into the iridium-carbon bond, step (3) to (4), results in the formation of a square pyramidal species with a bound acetyl ligand.
The active catalyst species (1) is regenerated by the reductive elimination of acetyl iodide from (4), a de-insertion reaction.