Lithium peroxide
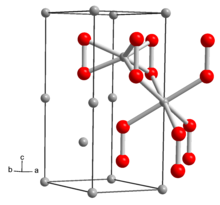
Dehydration of this material gives the anhydrous peroxide salt: Li2O2 decomposes at about 450 °C to give lithium oxide: The structure of solid Li2O2 has been determined by X-ray crystallography and density functional theory.
The solid features eclipsed "ethane-like" Li6O2 subunits with an O-O distance of around 1.5 Å.
[6] It is used in air purifiers where weight is important, e.g., spacecraft or other sealed spaces and apparatuses to absorb carbon dioxide and release oxygen in the reaction:[4] Li2O2 + CO2 → Li2CO3 + 1⁄2 O2 Similar to the reaction of lithium hydroxide with carbon dioxide to release 1 Li2CO3 and 1 H2O, lithium peroxide has high absorption capacity and absorbs more CO2 than does the same weight of lithium hydroxide and offers the bonus of releasing oxygen instead of water.
[7] Lithium peroxide can also act as a catalyst for polymerization of styrene to polystyrene.
The reversible lithium peroxide reaction is the basis for a prototype lithium–air battery.