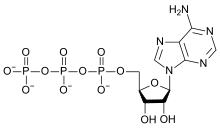
Oxygen compounds
Oxygen is reactive and will form oxides with all other elements except the noble gases helium, neon, argon and krypton.
[2] These hydrogen bonds between water molecules hold them approximately 15% closer than what would be expected in a simple liquid with just Van der Waals forces.
[3][4] Due to its electronegativity, oxygen forms chemical bonds with almost all other free elements at elevated temperatures to give corresponding oxides.
Oxygen is present as compounds in the atmosphere in trace quantities in the form of carbon dioxide (CO2) and oxides of nitrogen (NOx).
The rest of the Earth's crust is formed also of oxygen compounds, most importantly calcium carbonate (in limestone) and silicates (in feldspars).
[7] Oxygenated anions such as chlorates (ClO−3), perchlorates (ClO−4), chromates (CrO2−4), dichromates (Cr2O2−7), permanganates (MnO−4), and nitrates (NO−3) are strong oxidizing agents.
One unexpected oxygen compound is dioxygenyl hexafluoroplatinate, O+2PtF−6, discovered in studying the properties of platinum hexafluoride (PtF6).
[9] Among the most important classes of organic compounds that contain oxygen are (where "R" is an organic group): alcohol (R-OH); ethers (R-O-R); ketones (R-CO-R); aldehydes (R-CO-H); carboxylic acids (R-COOH); esters (R-COO-R); acid anhydrides (R-CO-O-CO-R); amides (R-C(O)-NR2).
Other important organic compounds that contain oxygen are: glycerol, formaldehyde, glutaraldehyde, citric acid, acetic anhydride, acetamide, etc.
Oxygen reacts spontaneously with many organic compounds at or below room temperature in a process called autoxidation.


2 O
3 , form when oxygen combines with other elements

2 )
(oxygen is in red, carbon in black and hydrogen in white)