Lutetium(III) iodide
Lutetium(III) iodide can be obtained by reacting lutetium with iodine:[5][2] Lutetium(III) iodide can also obtained by the reacting metallic lutetium with mercury iodide in vacuum at 500 °C:[5] The elemental mercury generated in the reaction can be removed by distillation.
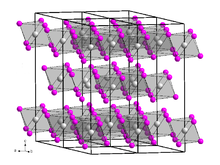
[7][5] It is a brown, very hygroscopic solid with a bismuth(III) iodide-type crystal structure.
In air, it quickly absorbs moisture and forms hydrates.
[2][3][4] The corresponding oxide iodide is also readily formed at elevated temperature.
[5] Lutetium(III) iodide doped with cerium is designed for use in PET scanners.