Lutetium tantalate
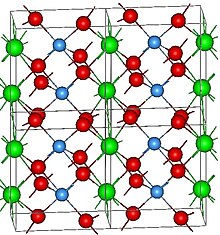
[2][3] Under standard conditions, LuTaO4 has a monoclinic (labeled as M'; Pearson symbol mP12, Space group = P2/a, No 13) fergusonite-type crystal structure.
[4] In the M' structure, the lutetium atom is 8-fold coordinated with oxygen and forms a distorted antiprism with a C2 site symmetry.
The high density of LuTaO4 favors X-ray excitation, which has relatively more efficient, stronger absorption in LuTaO4, compared to other materials.
To prepare a phosphor, a small fraction of appropriate material, such as an oxide of another rare-earth metal, is added to the mixture before annealing.
After cooling, the product is leached with water, washed, filtered and dried, resulting in a white powder consisting of micrometre-sized particles of LuTaO4.