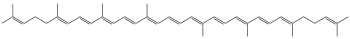
Lycopene
[4] In plants, algae, and other photosynthetic organisms, lycopene is an intermediate in the biosynthesis of many carotenoids, including beta-carotene, which is responsible for yellow, orange, or red pigmentation, photosynthesis, and photoprotection.
In its natural, all-trans form, the molecule is long and somewhat flat, constrained by its system of 11 conjugated double bonds.
[4] When exposed to light or heat, lycopene can undergo isomerization to any of a number of cis-isomers, which have a less linear shape.
[8][9] In human blood, various cis-isomers constitute more than 60% of the total lycopene concentration, but the biological effects of individual isomers have not been investigated.
[4] They are responsible for the bright orange–red colors of fruits and vegetables, perform various functions in photosynthesis, and protect photosynthetic organisms from excessive light damage.
[13] The unconditioned biosynthesis of lycopene in eukaryotic plants and in prokaryotic cyanobacteria is similar, as are the enzymes involved.
Two molecules of this product are then condensed in a tail-to-tail configuration to give the 40-carbon phytoene, the first committed step in carotenoid biosynthesis.
The two terminal isoprene groups of lycopene can be cyclized to produce beta-carotene, which can then be transformed into a wide variety of xanthophylls.
[20] Unlike other fruits and vegetables, where nutritional content such as vitamin C is diminished upon cooking, processing of tomatoes increases the concentration of bioavailable lycopene.
[4][23] Cooking and crushing tomatoes (as in the canning process) and serving in oil-rich dishes (such as spaghetti sauce or pizza) greatly increases assimilation from the digestive tract into the bloodstream.
[4] There are cases of intolerance or allergic reaction to dietary lycopene, which may cause diarrhea, nausea, stomach pain or cramps, gas, and loss of appetite.
[31] Other reviews concluded that research has been insufficient to establish whether lycopene consumption affects human health.
[34] The US Food and Drug Administration, in rejecting manufacturers' requests in 2005 to allow "qualified labeling" for lycopene and the reduction of various cancer risks, provided a conclusion that remains in effect as of 2015[update]: no studies provided information about whether lycopene intake may reduce the risk of any of the specific forms of cancer.