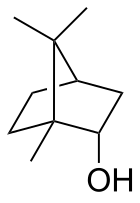
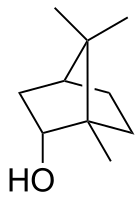
Borneol
Being chiral, borneol exists as enantiomers, both of which are found in nature: d-borneol (also written (+)-borneol) and l-borneol (or (−)-borneol).
[2] Borneol can be found in several species of Heterotheca,[3] Artemisia, Rosmarinus officinalis (rosemary)[4] Dipterocarpaceae, Blumea balsamifera and Kaempferia galanga.
Exposure to higher levels or over a longer period of time may cause restlessness, difficulty concentrating, irritability, and seizures.
[17] Borneol has been shown to have little to no irritation effect when applied to the human skin at doses used in fine fragrance formulation.
[18] Skin exposure can lead to sensitization and a future allergic reaction even to small quantities.
[17] The bornyl group is a univalent radical C10H17 derived from borneol by removal of hydroxyl and is also known as 2-bornyl.
[20] The structural isomer fenchol is also a widely used compound derived from certain essential oils.