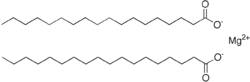
Magnesium stearate
It is a soap, consisting of salt containing two equivalents of stearate (the anion of stearic acid) and one magnesium cation (Mg2+).
[1][2] Magnesium stearate is often used as an anti-adherent[3] in the manufacture of medical tablets, capsules and powders.
[7][8][9] In the production of pressed candies, magnesium stearate serves as a release agent.
Magnesium stearate is generally considered safe for human consumption at levels below 2500 mg per kg of body weight per day[12] and is classified in the United States as generally recognized as safe (GRAS).
In 1979, the FDA's Subcommittee on GRAS Substances (SCOGS) reported, "There is no evidence in the available information on ... magnesium stearate ... that demonstrates, or suggests reasonable grounds to suspect, a hazard to the public when they are used at levels that are now current and in the manner now practiced, or which might reasonably be expected in the future.