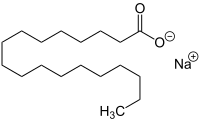
Sodium stearate
It is found in many types of solid deodorants, rubbers, latex paints, and inks.
[1] Having the characteristics of soaps, sodium stearate has both hydrophilic and hydrophobic parts, a carboxylate and a long hydrocarbon chain.
It is also used in the pharmaceutical industry as a surfactant to aid the solubility of hydrophobic compounds in the production of various mouth foams.
Sodium stearate is produced as a major component of soap upon saponification of oils and fats.
Tallow is especially high in stearic acid content (as the triglyceride), whereas most fats only contain a few percent.