Magnetochemistry
Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound.
For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula.
Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.
For chemical applications the molar magnetic susceptibility (χmol) is the preferred quantity.
This means that the effects are additive, and a table of "diamagnetic contributions", or Pascal's constants, can be put together.
[9] A metal ion with a single unpaired electron, such as Cu2+, in a coordination complex provides the simplest illustration of the mechanism of paramagnetism.
In the first-order Zeeman effect the energy difference between the two states is proportional to the applied field strength.
Denoting the energy difference as ΔE, the Boltzmann distribution gives the ratio of the two populations as
When the Curie law is obeyed, the product of molar susceptibility and temperature is a constant.
[12] For substances that obey the Curie law, the effective magnetic moment is independent of temperature.
It arises from a second-order Zeeman effect in which additional splitting, proportional to the square of the field strength, occurs.
It is difficult to observe as the compound inevitably also interacts with the magnetic field in the diamagnetic sense.
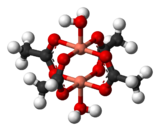
One of the simplest systems to exhibit the result of exchange interactions is crystalline copper(II) acetate, Cu2(OAc)4(H2O)2.
The state with spins opposed has lower energy, so the interaction can be classed as antiferromagnetic in this case.
[14] It is believed that this is an example of superexchange, mediated by the oxygen and carbon atoms of the acetate ligands.
They give rise to ferromagnetism, antiferromagnetism or ferrimagnetism, depending on the nature and relative orientations of the individual spins.
The total spin angular momentum is simply half the number of unpaired electrons and the spin-only formula results.
The spin-only formula is a good first approximation for high-spin complexes of first-row transition metals.
[21] The small deviations from the spin-only formula may result from the neglect of orbital angular momentum or of spin–orbit coupling.
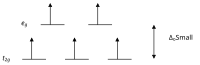
If the splitting is large enough to overcome the energy needed to place electrons in the same orbital, with opposite spin, a low-spin complex will result.
The effective moment varies from a typical d5 low-spin value of 2.25 μB at 80 K to more than 4 μB above 300 K.[23] Crystal field splitting is larger for complexes of the heavier transition metals than for the transition metals discussed above.
For example, uranium(IV), f2, in the complex [UCl6]2− has a measured effective moment of 2.2 μB, which includes a contribution from temperature-independent paramagnetism.
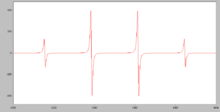
However, if the radicals are well separated from each other in a dilute solution in a solid matrix, at low temperature, they can be studied by electron paramagnetic resonance (EPR).
The simulated spectrum of the CH3• radical shows hyperfine splitting due to the interaction of the electron with the 3 equivalent hydrogen nuclei, each of which has a spin of 1/2.
[27][28] Spin labels are long-lived free radicals which can be inserted into organic molecules so that they can be studied by EPR.
[29] For example, the nitroxide MTSL, a functionalized derivative of TEtra Methyl Piperidine Oxide, TEMPO, is used in site-directed spin labeling.
[30] The magnetic moments of gadolinium compounds are larger than those of any transition metal ion.
Gadolinium is preferred to other lanthanide ions, some of which have larger effective moments, due to its having a non-degenerate electronic ground state.
Deoxy-hemoglobin is generally accepted to be a complex of iron in the +2 oxidation state, that is a d6 system with a high-spin magnetic moment near to the spin-only value of 4.9 μB.
The non-existence of Ga(II) compounds was part of the so-called inert-pair effect.