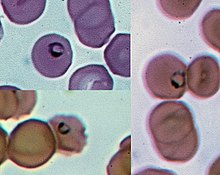
Plasmodium
Parasites grow within a vertebrate body tissue (often the liver) before entering the bloodstream to infect red blood cells.
During this infection, some parasites are picked up by a blood-feeding insect (mosquitoes in majority cases), continuing the life cycle.
Proteins are trafficked from the ER to the Golgi apparatus which generally consists of a single membrane-bound compartment in Apicomplexans.
[4] Like other apicomplexans, Plasmodium species have several cellular structures at the apical end of the parasite that serve as specialized organelles for secreting effectors into the host.
[5] Adjacent to the rhoptries are smaller structures termed micronemes that contain parasite proteins required for motility as well as recognizing and attaching to host cells.
[6] Species of Plasmodium also contain two large membrane-bound organelles of endosymbiotic origin, the mitochondrion and the apicoplast, both of which play key roles in the parasite's metabolism.
[7] Like in other eukaryotes, the Plasmodium mitochondrion is capable of generating energy in the form of ATP via the citric acid cycle; however, this function is only required for parasite survival in the insect host, and is not needed for growth in red blood cells.
[7] A second organelle, the apicoplast, is derived from a secondary endosymbiosis event, in this case the acquisition of a red alga by the Plasmodium ancestor.
[8] The apicoplast is involved in the synthesis of various metabolic precursors, including fatty acids, isoprenoids, iron-sulphur clusters, and components of the heme biosynthesis pathway.
[11] At this point, some species of Plasmodium of primates can form a long-lived dormant stage called a hypnozoite,[12] which can remain in the liver for more than a year.
They then go through continuous cycles of erythrocyte infection, while a small percentage of parasites differentiate into a sexual stage called a gametocyte which is picked up by an insect host taking a blood meal.
[10] Even when humans have such subclinical plasmodial infections, there can nevertheless be very large numbers of multiplying parasites concealed in, particularly, the spleen and bone marrow.
[11] Plasmodium belongs to the phylum Apicomplexa, a taxonomic group of single-celled parasites with characteristic secretory organelles at one end of the cell.
[17] The genus Plasmodium consists of over 200 species, generally described on the basis of their appearance in blood smears of infected vertebrates.
[19] The remaining subgenera: Asiamoeba, Carinamoeba, Lacertamoeba, Ophidiella, Paraplasmodium, and Sauramoeba contain the diverse groups of parasites found to infect reptiles.
[20] More recent studies of Plasmodium species using molecular methods have implied that the group's evolution has not perfectly followed taxonomy.
[17] There has been particular interest in dating the divergence of the human parasite P. falciparum from other Plasmodium lineages due to its medical importance.
[32] Resistance to quinine spurred the development of a broad array of antimalarial medications through the 20th century including chloroquine, proguanil, atovaquone, sulfadoxine/pyrimethamine, mefloquine, and artemisinin.
Mosquitoes of the genera Culex, Anopheles, Culiseta, Mansonia and Aedes act as insect hosts for various Plasmodium species.
[41][notes 1] The fact that several species may be involved in causing different forms of malaria was first recognized by Camillo Golgi in 1886.
[40] The contribution of insect hosts to the Plasmodium life cycle was described in 1897 by Ronald Ross and in 1899 by Giovanni Batista Grassi, Amico Bignami and Giuseppe Bastianelli.
[40] In 1966, Cyril Garnham proposed separating Plasmodium into nine subgenera based on host specificity and parasite morphology.
[42][19] This scheme was expanded upon by Sam R. Telford in 1988 when he reclassified Plasmodium parasites that infect reptiles, adding five subgenera.