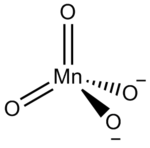
Manganate
In inorganic nomenclature, a manganate is any negatively charged molecular entity with manganese as the central atom.
[5] Manganates are dark green in colour, with a visible absorption maximum of λmax = 606 nm (ε = 1710 dm3 mol−1 cm−1).
[8] Sodium and potassium manganates are usually prepared in the laboratory by stirring the equivalent permanganate in a concentrated solution (5–10 M) of the hydroxide for 24 hours[6] or with heating.
However, its second acid dissociation constant has been estimated by pulse radiolysis techniques:[3] The name "manganite" is used for compounds formerly believed to contain the anion MnO3−3, with manganese in the +3 oxidation state.
However, most of these "manganites" do not contain discrete oxyanions, but are mixed oxides with perovskite (LaMnIIIO3, CaMnIVO3), spinel (LiMnIII,IV2O4) or sodium chloride (LiMnIIIO2, NaMnIIIO2) structures.