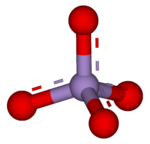
Permanganate
Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent.
[2] Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media.
[3] Permanganate compounds are common and strong disinfectants, used regularly to sanitize baths, toilets, and wash basins.
For instance, potassium permanganate decomposes at 230 °C to potassium manganate and manganese dioxide, releasing oxygen gas: In an acidic solution, permanganate(VII) is reduced to the pale pink manganese(II) (Mn2+) with an oxidation state of +2.
In a strongly basic or alkaline solution, permanganate(VII) is reduced to the green manganate ion, MnO2−4 with an oxidation state of +6.