Melanocortin 1 receptor
It controls the type of melanin being produced, and its activation causes the melanocyte to switch from generating the yellow-red phaeomelanin by default to the brown-black eumelanin in replacement.
[10] When activated by one of the variants of MSH, typically α-MSH, MC1R initiates a complex signaling cascade that leads to the production of eumelanin.
In contrast, the receptor can also be antagonized by agouti signalling peptide (ASIP), which reverts the cell back to producing the yellow or red phaeomelanin.
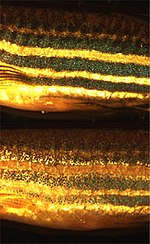
The yellow and black agouti banding pattern observed on most mammalian hair is caused by the pulsative nature of ASIP signalling through MC1R.
Exceptions include particoloured bay horses, which have reddish bodies, and black legs, mane, and tail, where ASIP signaling is limited to regions instead of pulsating.
[15] These observations suggest a role for mammalian MC1R outside the pigment cell, though the exact mechanism through which the protein can modulate pain sensation is not known.
In a certain genetic background in mice it has been reported that animals lacking MC1R had increased tolerance to capsaicin acting through the TRPV1 receptor and decreased response to chemically induced inflammatory pain.
[17] Lidocaine was reported to be much less effective in reducing pain in another study of humans with MC1R mutations[18] Since G protein–coupled receptors are known to activate Signal transduction in cells, it should not be surprising to find MC1R involved in development.
[21] These authors suggest that MC1R is important in anti-fungal and anti-inflammatory processes, in part because siRNA knockdown of MC1R almost completely prevented the responses.
In a rat model of nephritis it was found that treatment with a different agonist of MC1R improved aspects of kidney morphology and reduced proteinuria,[27][28] which may help explain the benefit of ACTH in humans.
The role of ASIP's binding to MC1R in regulating this adaptation is unclear; however, in teleost fish at least, functional antagonism is provided by melanin-concentrating hormone.
[29] Cephalopods generate a similar, albeit more dramatic, pigmentary effect using muscles to rapidly stretch and relax their pigmented chromatophores.
As humans migrated north, the absence of high levels of solar radiation in northern Europe and Asia relaxed the selective pressure on active MC1R, allowing the gene to mutate into dysfunctional variants without reproductive penalty, then propagate by genetic drift.