Hydrogen peroxide
It decomposes slowly into water and elemental oxygen when exposed to light, and rapidly in the presence of organic or reactive compounds.
Hydrogen peroxide (H2O2) is a nonplanar molecule with (twisted) C2 symmetry; this was first shown by Paul-Antoine Giguère in 1950 using infrared spectroscopy.
[11] These barriers are proposed to be due to repulsion between the lone pairs of the adjacent oxygen atoms and dipolar effects between the two O–H bonds.
It has been proposed that the enantiospecific interactions of one rather than the other may have led to amplification of one enantiomeric form of ribonucleic acids and therefore an origin of homochirality in an RNA world.
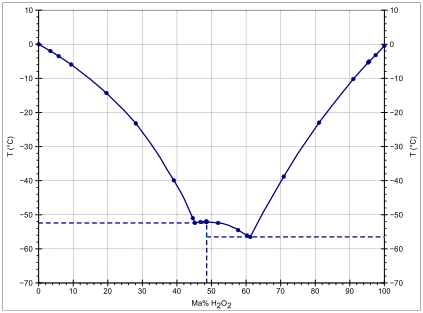
Its melting point is also fairly high, being comparable to that of hydrazine and water, with only hydroxylamine crystallising significantly more readily, indicative of particularly strong hydrogen bonding.
[17] Concentrations in air are about 0.4 to 4 μg/m3, varying over several orders of magnitude depending in conditions such as season, altitude, daylight and water vapor content.
[26] H2O=O seemed to be just as possible as the modern structure, and as late as in the middle of the 20th century at least half a dozen hypothetical isomeric variants of two main options seemed to be consistent with the available evidence.
[31][clarification needed] Today, hydrogen peroxide is manufactured almost exclusively by the anthraquinone process, which was originally developed by BASF in 1939.
[37] Hydrogen peroxide disproportionates to form water and oxygen with a ΔHo of –2884.5 kJ/kg[38] and a ΔS of 70.5 J/(mol·K): The rate of decomposition increases with rise in temperature, concentration, and pH.
[49] In addition, when excess H2O2 accumulates in the cell, catalase converts it to H2O through this reaction: Glutathione peroxidase, a selenoenzyme, also catalyzes the disproportionation of hydrogen peroxide.
[52][53][54] The compound is a major factor implicated in the free-radical theory of aging, based on its ready conversion into a hydroxyl radical.
The bombardier beetle combines hydroquinone and hydrogen peroxide, leading to a violent exothermic chemical reaction to produce boiling, foul-smelling liquid that partially becomes a gas (flash evaporation) and is expelled through an outlet valve with a loud popping sound.
Hydrogen peroxide reacts with certain di-esters, such as phenyl oxalate ester (cyalume), to produce chemiluminescence; this application is most commonly encountered in the form of glow sticks.
The propellant is pumped into a reaction chamber, where a catalyst, usually a silver or platinum screen, triggers decomposition, producing steam at over 600 °C (1,100 °F), which is expelled through a nozzle, generating thrust.
Hydrazine eventually replaced hydrogen peroxide monopropellant thruster applications primarily because of a 25% increase in the vacuum specific impulse.
[citation needed] The Bell Rocket Belt, reaction control systems for X-1, X-15, Centaur, Mercury, Little Joe, as well as the turbo-pump gas generators for X-1, X-15, Jupiter, Redstone and Viking used hydrogen peroxide as a monopropellant.
Operator error in the use of hydrogen peroxide torpedoes was named as possible causes for the sinking of HMS Sidon and the Russian submarine Kursk.
This torpedo, used by the Swedish Navy, is powered by a piston engine propelled by HTP as an oxidizer and kerosene as a fuel in a bipropellant system.
[further explanation needed] Hydrogen peroxide may be mixed with baking soda and salt to make a homemade toothpaste.
Hydrogen peroxide may be used in combination with a UV-light source to remove yellowing from white or light grey acrylonitrile butadiene styrene (ABS) plastics to partially or fully restore the original color.
In high concentrations, hydrogen peroxide is an aggressive oxidizer and will corrode many materials, including human skin.
The EPA Reportable Quantity (RQ) for D001 hazardous wastes is 100 pounds (45 kg), or approximately 10 US gallons (38 L), of concentrated hydrogen peroxide.
[101] The U.S. Occupational Safety and Health Administration (OSHA) has established a permissible exposure limit of 1.0 ppm calculated as an 8-hour time-weighted average (29 CFR 1910.1000, Table Z-1).
[97] Hydrogen peroxide has been classified by the American Conference of Governmental Industrial Hygienists (ACGIH) as a "known animal carcinogen, with unknown relevance on humans".
[103] Historically, hydrogen peroxide was used for disinfecting wounds, partly because of its low cost and prompt availability compared to other antiseptics.
[107] Practitioners of alternative medicine have advocated the use of hydrogen peroxide for various conditions, including emphysema, influenza, AIDS, and in particular cancer.
Free hydrogen peroxide will damage any tissue it encounters via oxidative stress, a process that also has been proposed as a cause of cancer.
It is also difficult to raise the level of oxygen around cancer cells within a tumour, as the blood supply tends to be poor, a situation known as tumor hypoxia.
Large oral doses of hydrogen peroxide at a 3% concentration may cause irritation and blistering to the mouth, throat, and abdomen as well as abdominal pain, vomiting, and diarrhea.
[109] Ingestion of hydrogen peroxide at concentrations of 35% or higher has been implicated as the cause of numerous gas embolism events resulting in hospitalisation.