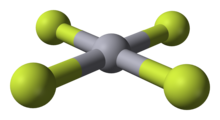
Mercury(IV) fluoride
[1][2] Speculation about higher oxidation states for mercury had existed since the 1970s, and theoretical calculations in the 1990s predicted that it should be stable in the gas phase, with a square-planar geometry consistent with a formal d8 configuration.
However, experimental proof remained elusive until 2007, when HgF4 was first prepared using solid neon and argon for matrix isolation at a temperature of 4 K. The compound was detected using infrared spectroscopy.
[5] Theoretical studies suggest that mercury is unique among the natural elements of group 12 in forming a tetrafluoride, and attribute this observation to relativistic effects.
According to calculations, the tetrafluorides of the "less relativistic" elements cadmium and zinc are unstable and eliminate a fluorine molecule, F2, to form the metal difluoride complex.
[7] Chemical historian William B. Jensen has argued that the compound alone is insufficient to reclassify the metal, because HgF4 represents at best a non-equilibrium transient state.