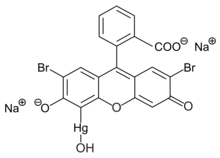
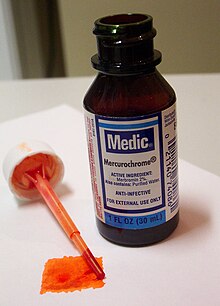
Merbromin
Readily available in most countries, it is no longer sold in Switzerland, Brazil, France, Iran, Germany, Denmark, or the United States, due to its mercury content.
In 1998, the U.S. Food and Drug Administration reclassified merbromin from "generally recognized as safe" to "untested," due to a lack of recent studies or updated supporting information.
The name is also commonly used for over-the-counter antiseptic solutions consisting of merbromin (typically at 2% concentration) dissolved in either ethyl alcohol (tincture) or water (aqueous).
On 19 October 1998, citing potential for mercury poisoning, the US Food and Drug Administration (FDA) reclassified merbromin from "generally recognized as safe" to "untested," effectively halting its distribution within the United States.
[citation needed] Within the United States, products such as Humco Mercuroclear ("Aqueous solution of benzalkonium chloride and lidocaine hydrochloride") play on the brand recognition history of Mercurochrome but substitute other ingredients with similar properties.