Methylenetriphenylphosphorane
It is the parent member of the phosphorus ylides, popularly known as Wittig reagents.
Methylenetriphenylphosphorane is prepared from methyltriphenylphosphonium bromide by its deprotonation using a strong base like butyllithium:[1] The phosphorane is generally not isolated, instead it is used in situ.
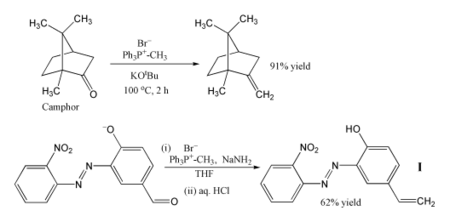
[4] Methylenetriphenylphosphorane is used to replace oxygen centres in aldehydes and ketones with a methylene group, i.e., a methylenation: The phosphorus-containing product is triphenylphosphine oxide.
Crystallographic characterization of the colourless ylide reveals that the phosphorus atom is approximately tetrahedral.
[5] The compound is usually described as a combination of two resonance structures: Methylenetriphenylphosphorane has become a standard tool for synthetic organic chemists.