Montmorillonite
Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay.
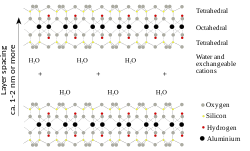
Montmorillonite is a subclass of smectite, a 2:1 phyllosilicate mineral characterized as having greater than 50% octahedral charge; its cation exchange capacity is due to isomorphous substitution of Mg for Al in the central alumina plane.
In contrast, beidellite is smectite with greater than 50% tetrahedral charge originating from isomorphous substitution of Al for Si in the silica sheet.
Chemically, it is hydrated sodium calcium aluminium magnesium silicate hydroxide (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O.
Montmorillonites expand considerably more than other clays due to water penetrating the interlayer molecular spaces and concomitant adsorption.
Hence, sodium montmorillonite has come to be used as the major constituent in nonexplosive agents for splitting rock in natural stone quarries in an effort to limit the amount of waste, or for the demolition of concrete structures where the use of explosive charges is unacceptable.
[citation needed] This swelling property makes montmorillonite-containing bentonite useful also as an annular seal or plug for water wells and as a protective liner for landfills.
Other uses include as an anticaking agent in animal feed, in papermaking to minimize deposit formation, and as a retention and drainage aid component.
[12] Montmorillonite clay is added to some dog and cat foods as an anti-caking agent and because it may provide some resistance to environmental toxins, though research on the subject is not yet conclusive.