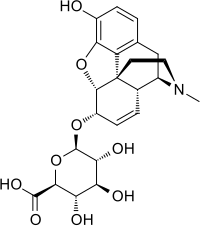
Morphine-6-glucuronide
[1] It has analgesic effects roughly half that of morphine.
[5] Subsequent work at St Bartholomew's Hospital, London in the 1980s,[6] using a sensitive and specific high-performance liquid chromatography assay,[7] accurately defined for the first time the metabolism of morphine, and the abundance of this metabolite (along with morphine-3-glucuronide,[8] considered an inactive metabolite).
It was postulated that kidney impairment would result in accumulation of the kidney-excreted active agent M6G, leading to potentially fatal toxicity such as respiratory depression.
The frequent use of morphine in critically ill patients, and the common occurrence of kidney failure in this group implied that M6G accumulation could be a common, but previously unanticipated problem.
[4] A key step in defining the importance of M6G in humans came in 1992 when the substance was artificially synthesised and administered to patients with pain, the majority of whom described pain relief.