Morphine
[35][36] Morphine is also available as a slow-release formulation for opiate substitution therapy (OST) in Austria, Germany, Bulgaria, Slovenia, and Canada for persons with opioid addiction who cannot tolerate either methadone or buprenorphine.
The findings from this study suggest that stable opioid use does not significantly impair abilities inherent in driving (this includes physical, cognitive, and perceptual skills).
COAT patients showed rapid completion of tasks that require the speed of responding for successful performance (e.g., Rey Complex Figure Test) but made more errors than controls.
It is important to note that this study reveals that COAT patients have no domain-specific deficits, which supports the notion that chronic opioid use has minor effects on psychomotor, cognitive, or neuropsychological functioning.
Cessation of dosing with morphine creates the prototypical opioid withdrawal syndrome, which, unlike that of barbiturates, benzodiazepines, alcohol, or sedative-hypnotics, is not fatal by itself in otherwise healthy people.
Early symptoms include watery eyes, insomnia, diarrhea, runny nose, yawning, dysphoria, sweating, and, in some cases, a strong drug craving.
Addicts often experience severe depression, anxiety, insomnia, mood swings, forgetfulness, low self-esteem, confusion, paranoia, and other psychological problems.
[67][68] The μ-binding sites are discretely distributed in the human brain, with high densities in the posterior amygdala, hypothalamus, thalamus, nucleus caudatus, putamen, and certain cortical areas.
Morphine is subject to extensive first-pass metabolism (a large proportion is broken down in the liver), so, if taken orally, only 40% to 50% of the dose reaches the central nervous system.
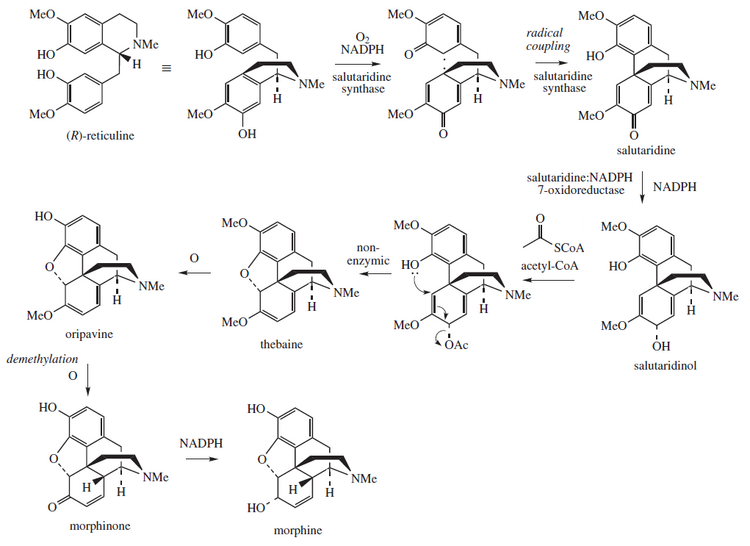
[92][93] Morphine is the most abundant opiate found in opium, the dried latex extracted by shallowly scoring the unripe seedpods of the Papaver somniferum poppy.
[94] Przemko and Norman cultivars of the opium poppy, are used to produce two other alkaloids, thebaine and oripavine, which are used in the manufacture of semi-synthetic and synthetic opioids like oxycodone and etorphine.
[15][99] Urinary concentrations of endogenous codeine and morphine have been found to significantly increase in individuals taking L-DOPA for the treatment of Parkinson's disease.
[104][105] Elements of the morphine structure have been used to create completely synthetic drugs such as the morphinan family (levorphanol, dextromethorphan and others) and other groups that have many members with morphine-like qualities.
[106] As Jack DeRuiter of the Department of Drug Discovery and Development (formerly, Pharmacal Sciences), Harrison School of Pharmacy, Auburn University stated in his Fall 2000 course notes for that earlier department's "Principles of Drug Action 2" course, "Examination of the morphine molecule reveals the following structural features important to its pharmacological profile...Morphine and most of its derivatives do not exhibit optical isomerism, although some more distant relatives like the morphinan series (levorphanol, dextrorphan, and the racemic parent chemical racemorphan) do,[108] and as noted above stereoselectivity in vivo is an important issue.
[citation needed] As a result of the extensive study and use of this molecule, more than 250 morphine derivatives (also counting codeine and related drugs) have been developed since the last quarter of the 19th century.
[citation needed] Morphine ascorbate and other salts such as the tannate, citrate, and acetate, phosphate, valerate and others may be present in poppy tea depending on the method of preparation.
The somewhat similar Gregory process was developed in the United Kingdom during the Second World War, which begins with stewing the entire plant, in most cases save the roots and leaves, in plain or mildly acidified water, then proceeding through steps of concentration, extraction, and purification of alkaloids.
[citation needed] Other methods of processing "poppy straw" (i.e., dried pods and stalks) use steam, one or more of several types of alcohol, or other organic solvents.
However, in Turkey and Tasmania, morphine is obtained by harvesting and processing the fully mature dry seed pods with attached stalks, called poppy straw.
It was announced in 1973 that a team at the National Institutes of Health in the United States had developed a method for total synthesis of morphine, codeine, and thebaine using coal tar as a starting material.
[115] Several other syntheses were reported, notably by the research groups of Rice,[116] Evans,[117] Fuchs,[118] Parker,[119] Overman,[120] Mulzer-Trauner,[121] White,[122] Taber,[123] Trost,[124] Fukuyama,[125] Guillou,[126] and Stork.
[127] Because of the stereochemical complexity and consequent synthetic challenge presented by this polycyclic structure, Michael Freemantle has expressed the view that it is "highly unlikely" that a chemical synthesis will ever be cost-effective such that it could compete with the cost of producing morphine from the opium poppy.
[129] Morphine is a precursor in the manufacture of several opioids such as dihydromorphine, hydromorphone, hydrocodone, and oxycodone as well as codeine, which itself has a large family of semi-synthetic derivatives.
[citation needed] Other clandestine conversions—of morphine, into ketones of the hydromorphone class, or other derivatives like dihydromorphine (Paramorfan), desomorphine (Permonid), metopon, etc., and of codeine into hydrocodone (Dicodid), dihydrocodeine (Paracodin), etc.
[134] Morphine was discovered as the first active alkaloid extracted from the opium poppy plant in December 1804 in Paderborn by German pharmacist Friedrich Sertürner.
However, Sertürner became addicted to the drug, warning that "I consider it my duty to attract attention to the terrible effects of this new substance I called morphium in order that calamity may be averted.
[139] Commercial production began in Darmstadt, Germany, in 1827 by the pharmacy that became the pharmaceutical company Merck, with morphine sales being a large part of their early growth.
[140][141] In the 1850s, Alexander Wood reported that he had injected morphine into his wife Rebecca as an experiment; the myth goes that this killed her because of respiratory depression,[136] but she outlived her husband by ten years.
[149] At least three methods of total synthesis of morphine from starting materials such as coal tar and petroleum distillates have been patented, the first of which was announced in 1952, by Marshall D. Gates, Jr. at the University of Rochester.
[14] Informal names for morphine include: Cube Juice, Dope, Dreamer, Emsel, First Line, God's Drug, Hard Stuff, Hocus, Hows, Lydia, Lydic, M, Miss Emma, Mister Blue, Monkey, Morf, Morph, Morphide, Morphie, Morpho, Mother, MS, Ms. Emma, Mud, New Jack Swing (if mixed with heroin), Sister, Tab, Unkie, Unkie White, and Stuff.