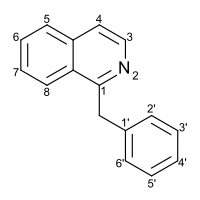
Benzylisoquinoline
Substitution of the heterocycle isoquinoline at the C1 position by a benzyl group provides 1‑benzylisoquinoline, the most widely examined of the numerous benzylisoquinoline structural isomers.
The 1-benzylisoquinoline moiety can be identified within numerous compounds of pharmaceutical interest, such as moxaverine; but most notably it is found within the structures of a wide variety of plant natural products, collectively referred to as benzylisoquinoline alkaloids.
These pathways collectively lead to the structurally disparate compounds comprising the broad classification of plant natural products referred to as benzylisoquinoline alkaloids (BIA), which have been comprehensively discussed by Hagel.
[3] The biosynthesis of (S)-norcoclaurine, which is catalyzed by (S)-norcoclaurine synthase, is accomplished by the stereoselective condensation of dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA); each of these compounds is prepared by multiple enzymatic transformations from L-tyrosine.
It is of interest to note that early studies initially identified norlaudanosoline (tetrahydropapaveroline) as the purported central precursor for the biosynthesis of BIAs.