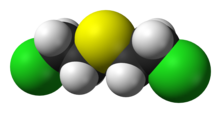
Mustard gas
[4] The name mustard gas is technically incorrect; the substances, when dispersed, are often not gases but a fine mist of liquid droplets that can be readily absorbed through the skin and by inhalation.
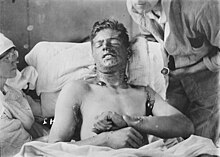
Extreme ocular exposure to mustard gas vapors may result in corneal ulceration, anterior chamber scarring, and neovascularization.
[3] In a study of patients 25 years after wartime exposure to chemical weaponry, c-DNA microarray profiling indicated that 122 genes were significantly mutated in the lungs and airways of mustard gas victims.
Those genes all correspond to functions commonly affected by mustard gas exposure, including apoptosis, inflammation, and stress responses.
[medical citation needed] For skin lesions, topical treatments, such as calamine lotion, steroids, and oral antihistamines are used to relieve itching.
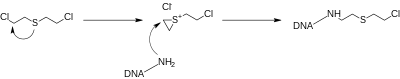
These very reactive intermediates tend to permanently alkylate nucleotides in DNA strands, which can prevent cellular division, leading to programmed cell death.
In 1860, the British scientist Frederick Guthrie synthesized and characterized the mustard agent compound and noted its irritating properties, especially in tasting.
In 1913, the English chemist Hans Thacher Clarke (known for the Eschweiler-Clarke reaction) replaced the phosphorus trichloride with hydrochloric acid in Meyer's formulation while working with Emil Fischer in Berlin.
[25] Mustard gas can have the effect of turning a patient's skin different colors, including shades of red, orange, pink, and in unusual cases, blue.
Mustard agent has also been dispersed in such munitions as aerial bombs, land mines, mortar rounds, artillery shells, and rockets.
The early countermeasures against mustard agent were relatively ineffective, since a soldier wearing a gas mask was not protected against absorbing it through his skin and being blistered.
A common countermeasure was using a urine-soaked mask or facecloth to prevent or reduce injury, a readily available remedy attested by soldiers in documentaries (e.g.
They Shall Not Grow Old in 2018) and others (such as forward aid nurses) interviewed between 1947 and 1981 by the British Broadcasting Corporation for various World War One history programs; however, the effectiveness of this measure is unclear.
An example of this was depicted in a British and Canadian documentary about life in the trenches, particularly once the "sousterrain" (subways and berthing areas underground) were completed in Belgium and France.
Towards the end of World War I, mustard agent was used in high concentrations as an area-denial weapon that forced troops to abandon heavily contaminated areas.
[49] In addition, autopsies performed on 75 soldiers who had died of mustard agent during World War I were done by researchers from the University of Pennsylvania who reported decreased counts of white blood cells.
[50] In a parallel track, after the air raid on Bari in December 1943, the doctors of the U.S. Army noted that white blood cell counts were reduced in their patients.
Some years after World War II was over, the incident in Bari and the work of the Yale University group with nitrogen mustard converged, and this prompted a search for other similar chemical compounds.
[53] In 1946, 10,000 drums of mustard gas (2,800 tonnes) stored at the production facility of Stormont Chemicals in Cornwall, Ontario, Canada, were loaded onto 187 boxcars for the 900 miles (1,400 km) journey to be buried at sea on board a 400 foot (120 m) long barge 40 miles (64 km) south of Sable Island, southeast of Halifax, at a depth of 600 fathoms (1,100 m).
[54] A large British stockpile of old mustard agent that had been made and stored since World War I at M. S. Factory, Valley near Rhydymwyn in Flintshire, Wales, was destroyed in 1958.
On mechanically breaking these lumps (e.g., with the drag board of a fishing net or by the human hand) the enclosed mustard gas is still as active as it had been at the time the weapon was dumped.
Artillery shells containing mustard gas and other toxic ammunition from World War I (as well as conventional explosives) can still be found in France and Belgium.
[61] A significant portion of the United States' mustard agent stockpile was stored at the Edgewood Area of Aberdeen Proving Ground in Maryland.
The nearest schools were fitted with overpressurization machinery to protect the students and faculty in the event of a catastrophic explosion and fire at the site.
[63] The largest mustard agent stockpile, at approximately 6,200 short tons, was stored at the Deseret Chemical Depot in northern Utah.
In 2008, many empty aerial bombs that contained mustard gas were found in an excavation at the Marrangaroo Army Base just west of Sydney, Australia.
[64][65] In 2009, a mining survey near Chinchilla, Queensland, uncovered 144 105-millimeter howitzer shells, some containing "Mustard H", that had been buried by the U.S. Army during World War II.
The experiments were classified secret and as with Agent Orange[broken anchor], claims for medical care and compensation were routinely denied, even after the WWII-era tests were declassified in 1993.
[74] African American servicemen were tested alongside white men in separate trials to determine whether their skin color would afford them a degree of immunity to the agents, and Nisei servicemen, some of whom had joined after their release from Japanese American Internment Camps were tested to determine susceptibility of Japanese military personnel to these agents.
The presence in urine of 1,1'-sulfonylbismethylthioethane (SBMTE), a conjugation product with glutathione, is considered a more specific marker, since this metabolite is not found in specimens from unexposed persons.