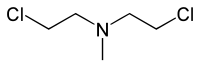
Nitrogen mustard
[6] In early December of 1943, an incident during the air raid on Bari, Italy, led to the release of mustard gas that affected several hundred soldiers and civilians.
[8] After World War II was over, the Bari incident and the Yale group's studies eventually converged prompting a search for other similar compounds.
[citation needed] The nitrogen mustard drug mustine (HN2), is no longer commonly in use in its original IV formulation because of excessive toxicity.
Their weapon designations are:[11] Normustard (mustine without a methyl group on the nitrogen atom; bis(2-chloroethyl)ethylamine) can be used in the synthesis of piperazine drugs such as mazapertine, aripiprazole & fluanisone.
A second attack after the displacement of the second chlorine atom forms the second alkylation step that results in the formation of interstrand cross-links (ICLs) as it was shown in the early 1960s.
[18] These kinds of lesions are effective at forcing the cell to undergo apoptosis via p53,[citation needed] a protein which scans the genome for defects.