Neisseria gonorrhoeae
Neisseria gonorrhoeae, also known as gonococcus (singular) or gonococci (plural), is a species of Gram-negative diplococci bacteria first isolated by Albert Neisser in 1879.
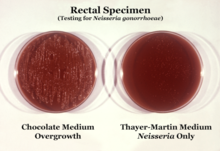
[3] An obligate human pathogen, it primarily colonizes the mucosal lining of the urogenital tract; however, it is also capable of adhering to the mucosa of the nose,[4] pharynx, rectum, and conjunctiva.
[21] Members of this genus do not form endospores and are nonmotile, with the exception of pathogenic species, which capable of moving using twitching motility;[22] most are also obligate aerobes.
[30] While this acetate can enter the CAC for further oxidation, this does not occur so long as other carbon sources such as glucose or lactate are present, in which case it is excreted from the cell or incorporated for lipid synthesis.
[27] N. gonorrhoeae, like other pathogenic members of the genus Neisseria, are capnophiles, meaning they require higher-than-normal concentrations of carbon dioxide (CO2) to grow , either in the form of CO2 or bicarbonate (HCO3−) depending on the bacterial strain.
[32] As an obligate human pathogen and a facultative anaerobic capnophile, Neisseria gonorrhoeae typically colonize mucosal surfaces in microaerobic environments, such as those in the genitourinary tract.
[34][35] The general purpose of the ETC is the formation of the electrochemical gradient of hydrogen ions (H+ or protons), resulting from concentration differences across the plasma membrane, needed to power ATP production in a process known as oxidative phosphorylation.
[39] The general purpose of the ETC is the formation of the electrochemical gradient of hydrogen ions (H+ or protons), resulting from concentration differences across the plasma membrane, needed to power ATP production in a process known as oxidative phosphorylation.
[19][40] Along with the sequestration defence that can be further upregulated by host inflammation, humans also produce siderocalins that are able to chelate siderophores to as a further method of inhibiting pathogenic bacterial growth.
[43] Dynamic polymeric protein filaments called type IV pili allow N. gonorrhoeae to do many bacterial processes including adhesion to surfaces, transformation competence, twitching motility, and immune response evasions.
[44] For motility, individual bacteria use their pili in a manner that resembles a grappling hook: first, they are extended from the cell surface and attach to a substrate.
It is a sugar (saccharide) side chain attached to lipid A (thus "lipo-") in the outer membrane coating the cell wall of the bacteria.
[9] These changes allow for adjustment to local environmental differences at the site of infection, evasion of recognition by targeted antibodies, and inhibit the formation of an effective vaccine.
[9] In addition to gene rearrangement, it is also naturally competent, meaning it can acquire extracellular DNA from the environment via its type IV pilus, specifically proteins PilQ and PilT.
[9] Frameshifts occur in both the pilE and pilC genes, effectively turning off the expression of pili in situations when they are not needed, such as during intracellular colonization as opposed to extracellular mucosal cell surface adhesion.
[50] However, a significant fraction of the gonococci can resist killing through the action of their catalase[6] which breaks down reactive oxygen species and is able to reproduce within the neutrophil phagosomes.
[58][59] Studies have identified that N. gonorrhoeae has obtained methods of antimicrobial resistance by way of horizontal gene transfer from other Neisseria species including N. lactamica, N. macacae, and N. mucosa.
[60] Transformation in N. gonorrhoeae is performed by the type IV pilus, where the DNA is bound and brought into the cell, followed by processing and homologous recombination.
[68][11] Disseminated gonococcal infections can occur when N. gonorrhoeae enters the bloodstream, often spreading to the joints and causing a rash (dermatitis-arthritis syndrome).
[68] Dermatitis-arthritis syndrome results in joint pain (arthritis), tendon inflammation (tenosynovitis), and painless non-pruritic (non-itchy) dermatitis.
[68][69] Gonococcal ophthalmia neonatorum, once common in newborns, is prevented by the application of erythromycin (antibiotic) gel to the eyes of babies at birth as a public health measure.
[7] Communal baths, shared towels or fabrics, rectal thermometers, and improper hand hygiene by caregivers have been identified as potential means of transmission in pediatric settings.
[72] Successful transmission is followed by adherence to the epithelial cells found at the infected mucosal site by the bacterium's type IV pili.
[41] During growth and colonization, N. gonorrhoeae stimulates the release of pro-inflammatory cytokines and chemokines from host immune cells that result in the recruitment of neutrophils to the area.
[75] If an individual is not allergic to cephalosporins but ceftriaxone is unavailable, an alternative treatment is a single dose of 800 mg cefixime consumed orally.
Antimicrobial resistance is not universal and N. gonorrhoeae strains in the United States continue to respond to a combination regimen of ceftriaxone and azithromycin.
[83] A cleaved portion of this protein, C3b, is deposited on pathogenic surfaces and results in opsonization as well as the downstream activation of the membrane attack complex.
[84][9] Thus, gonorrhea means "flow of seed", a description referring to the white penile discharge, assumed to be semen, seen in male infection.
[15] Then in 1883, Max Bockhart proved conclusively that the bacterium isolated by Albert Neisser was the causative agent of the disease known as gonorrhea by inoculating the penis of a healthy man with the bacteria.
[86] After Hunter's experiment other scientists sought to disprove his conclusions by inoculating other male physicians, medical students,[15] and incarcerated men with gonorrheal pus, who all developed the burning and discharge of gonorrhea.