NK1 receptor antagonist
The discovery of neurokinin 1 (NK1) receptor antagonists was a turning point in the prevention of nausea and vomiting associated with cancer chemotherapy.
The discovery and development of NK1 receptor antagonists have elicited antiemetic effect in both acute and especially in delayed phases of emesis.
A great effort was put into purifying this substance from diverse mammalian tissue, but 30 years of research were without success.
[7][9] Biological research that identified the many functions of tachykinins sparked interest in neurokinin receptor antagonists development.
Proposing the concept in the early 1990s, in 1998 Kramer, et al., reported clinical data on the efficacy and safety of MK-869 (aprepitant) in patients with major depressive disorder.
[1] In 2003, the first NK1 receptor antagonist, aprepitant (Emend), received marketing approval from the U.S. Food and Drug Administration (FDA).
The loops have functional sites, including two cysteines amino acids for a disulfide bridge, Asp-Arg-Tyr, which is responsible for association with arrestin and, Lys/Arg-Lys/Arg-X-X-Lys/Arg, which interacts with G-proteins.
SAR studies that were performed in order to improve the selectivity for the human NK1 receptor resulted in the development of a compound called RPR-100893.
This compound showed good activity in vivo and in models of pain and was developed up to phase II for the treatment of migraines but then terminated, as was the case with other NK1 receptor antagonists that were tested for the same indication.
CP-96345 has a rather simple structure, composed of a rigid quinuclidine scaffold containing a basic nitrogen atom, a benzhydril moiety and an o-methoxy-benzylamine group.
Strongly basic quinuclidine nitrogen on the compound was considered to be responsible for this Ca2+ binding, which caused a number of systemic effects, unrelated to the blocking of the NK1 receptor.
[17] CP-99994 eased dental pain in humans and entered phase II clinical trials; these were discontinued because of poor bioavailability.
It was developed up to phase II clinical trials for the treatment of postoperative nausea and vomiting, migraine and motion sickness.
It underwent phase IIa clinical trials for the treatment of osteoarthritis pain but showed no significant effects.
Eli Lilly made some SAR work on its structure and developed some compounds that did not enter clinical trials.
[12] By a general hypothesis on peptideric G protein-coupled receptors binding site, Takeda discovered a series of N-benzylcarboxyamides in 1995.
One of those compounds, TAK-637 (figure 6), underwent phase II clinical trials for urinary incontinence, depression and irritable bowel syndrome, but the development was discontinued.
The main ligand binding site is in the hydrophobic core between the loops and the outer segments of transmembrane domains 3–7 (TM3–TM7).
Each structural class of non-peptide NK1 receptor antagonists appears to interact with a specific set of residues within the common binding pocket.
[15] The bridgehead basic nitrogen is thought to interact with the NK1 receptor by mediating its recognition through ion pair site.
[20] It has been found that the basic nitrogen atoms in pyrido[3,4-b]pyridine do have an anchoring function in the phospholipid component of the cell membrane.
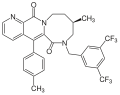
[15] In the development of MK-869, it was discovered that 3,5-disubstitution of the benzyl ring in the ether series gave greater potency than the 2-methoxy substitution in earlier benzylamine structures.
It also was revealed that the TFMP group appeared to be especially important, and it is believed that it enhances activity in vivo and improves metabolism.
[11] The presence of an intramolecular face-to-face π–π interaction between two aromatic rings is a common feature of high affinity NK1 receptor antagonists.