Nickel–cadmium battery
Compared with other types of rechargeable cells they offer good cycle life and performance at low temperatures with a fair capacity but their significant advantage is the ability to deliver practically their full rated capacity at high discharge rates (discharging in one hour or less).
Within the European Union, Ni–Cd batteries can now only be supplied for replacement purposes or for certain types of new equipment such as medical devices.
In 1906, Jungner established a factory close to Oskarshamn, Sweden, to produce flooded design Ni–Cd batteries.
In 1932, active materials were deposited inside a porous nickel-plated electrode and fifteen years later work began on a sealed nickel–cadmium battery.
Fusing nickel powder at a temperature well below its melting point using high pressures creates sintered plates.
Sintered plates are usually much thinner than the pocket type, resulting in greater surface area per volume and higher currents.
In general, the greater amount of reactive material surface area in a battery, the lower its internal resistance.
[citation needed] Model-aircraft or -boat builders often take much larger currents of up to a hundred amps or so from specially constructed Ni–Cd batteries, which are used to drive main motors.
While most pocket radios will operate satisfactorily at this voltage, some manufacturers such as Varta made 8.4 volt batteries with seven cells for more critical applications.
Sealed Ni–Cd cells consist of a pressure vessel that is supposed to contain any generation of oxygen and hydrogen gases until they can recombine back to water.
Since the vessel is designed to contain an exact amount of electrolyte this loss will rapidly affect the capacity of the cell and its ability to receive and deliver current.
To detect all conditions of overcharge demands great sophistication from the charging circuit and a cheap charger will eventually damage even the best quality cells.
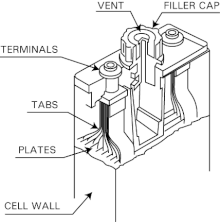
[5] A fully charged Ni–Cd cell contains: Ni–Cd batteries usually have a metal case with a sealing plate equipped with a self-sealing safety valve.
The positive and negative electrode plates, isolated from each other by the separator, are rolled in a spiral shape inside the case.
As a relatively small area of the electrode is in contact with the electrolyte (as opposed to the jelly-roll design), the internal resistance for an equivalent sized alkaline cell is higher which limits the maximum current that can be delivered.
The alkaline electrolyte (commonly KOH) is not consumed in this reaction and therefore its specific gravity, unlike in lead–acid batteries, is not a guide to its state of charge.
When Jungner built the first Ni–Cd batteries, he used nickel oxide in the positive electrode, and iron and cadmium materials in the negative.
This also means the battery is not normally damaged by excessive rates of overcharge, discharge or even negative charge.
Vented-cell Ni–Cd batteries have long lives (up to 20 years or more, depending on type) and operate at extreme temperatures (from −40 to 70 °C).
Cells are usually made of a light and durable polyamide (nylon), with multiple nickel–cadmium plates welded together for each electrode inside.
Large nickel-plated copper studs and thick interconnecting links assure minimum equivalent series resistance for the battery.
Miniature button cells are sometimes used in photographic equipment, hand-held lamps (flashlight or torch), computer-memory standby, toys, and novelties.
This makes them a favourable choice for remote-controlled electric model airplanes, boats, and cars, as well as cordless power tools and camera flash units.
Advances in battery-manufacturing technologies throughout the second half of the twentieth century have made batteries increasingly cheaper to produce.
A fully charged single Ni–Cd cell, under no load, carries a potential difference of between 1.25 and 1.35 volts, which stays relatively constant as the battery is discharged.
[10] Ni–Cd batteries may suffer from a "memory effect" if they are discharged and recharged to the same state of charge hundreds of times.
The original paper describing the memory effect was written by GE scientists at their Battery Business Department in Gainesville, Florida, and later retracted by them, but the damage was done.
This results from repeated overcharging; the symptom is that the battery appears to be fully charged but discharges quickly after only a brief period of operation.
In rare cases, much of the lost capacity can be recovered by a few deep-discharge cycles, a function often provided by automatic battery chargers.
[13] Under the same EU directive, used industrial Ni–Cd batteries must be collected by their producers in order to be recycled in dedicated facilities.